Al-Si diagram is a eutectic diagram where solid solubility at least of aluminium in silicon is negligible, and maximum solubility of silicon in aluminium (at eutectic temperature) is 1.65%. The eutectic composition is 12.7% silicon. Fig. 3.18 illustrates this diagram and photomicrographs of alloys at room temperature.
Alloys of Al-Si are very important casting alloys mainly because of high fluidity imparted due to large volumes of the Al-Si eutectic. Castings have high resistance to corrosion, good weldability, and silicon reduces the coefficient of thermal expansion.
The microstructure of 99.95% aluminium shows typical equiaxed grains of a pure metal. The microstructure of pure silicon also shows grains. The microstructure of Al-8% Si alloy shows dendrites of alpha solid solution (primary or proeutectic) in the eutectic mixture. Alpha is almost pure aluminium.
ADVERTISEMENTS:
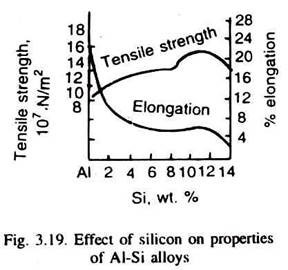
The primary, or proeutectic solid phase, which is almost pure Si or silicon-rich beta phase in Al-20% Si as well as in Al-50% Si alloys appears in geometric shapes. This is because silicon has predominantly non-metallic properties with diamond cubic crystal structure. In the eutectic mixture, silicon (or beta) -appears as acicular (needle like) or rod-shaped. Fig. 3.19 illustrates the variation of typical properties of cast aluminium-silicon alloys.
Slow solidification of pure Al-Si alloys produces a very coarse eutectic microstructure consisting of large plates or needles of silicon in a continuous aluminium matrix. The silicon particles appear to be interconnected.
ADVERTISEMENTS:
This coarse eutectic results in low ductility because of the brittle nature of large silicon plates. Permanent-mould castings having rapid cooling have greatly refined microstructure, where silicon phase assumes a fibrous form, which results in improvement in both ductility and tensile strength.
Modification of Al-Si alloys is commonly done as it results is refinement of microstructure, by adding ‘modifying agent’ to the melt prior to pouring as a mixture of sodium fluoride and sodium chloride (2/3:1/3), so that it has 0.005 to 0.015% residual metallic sodium.
The mechanical properties are improved due to refinement of microstructure, and even an original hypereutectic alloy solidifies as hypoeutectic alloy, Fig. 3.20. The remelting destroys the modification effects. If instead of sodium salt, strontium (0.03-0.05%) as Al-Sr. or Al-Si-Sr alloy when added retains the modified effects even on remelting. Table 3.1 illustrates effect of modification on mechanical properties.
Sodium depresses the eutectic temperature by as much as 12°C as illustrated in Fig. 3.18. The finer eutectic is produced because the rate of nucleation is higher in the super-cooled condition.
Probably by forming a film of sodium silicide (Na2Si) on silicon, sodium restricts the growth of silicon particles. Al-Si alloys like LM4. LM8 and LM25 find applications in all types of castings.