In this article we will discuss about:- 1. Introduction to Cast Irons 2. Composition and Cooling Rate of Cast Irons 3. Comparison of Properties 4. Science of Development of Microstructures.
Introduction to Cast Irons:
Cast irons are iron-carbon (and silicon) alloys having carbon or carbon equivalent value, more than 2% (actually it is 2.1 1%), i.e., more than the maximum solid solubility of carbon in austenite such that the eutectic reaction occurs during solidification. As the higher carbon contents make them more brittle, industrial cast irons have carbon normally in the range of 2.11 to 4% and silicon 0.5 to 3% (along-with other elements like manganese, sulphur and phosphorus).
Cast irons, being brittle, cannot be forged, rolled, drawn, etc., but can only be ‘cast’ into desired shapes and size (with, or without machining) by pouring the molten alloy of desired composition into a mould of desired shape and then, allowing it to solidify.
As casting is the only and exclusive suitable process to shape these alloys, these are called cast irons. Cast irons are least expensive, low melting (1140°-1200°C) materials with good castability, good machinability, good wear resistance, high damping capacity, high compressive strength (3-5 times of tensile strength), notch-insensitive (grey irons) and good corrosion and heat-resistance. Although cast irons are inferior to steel in mechanical properties, these are superior in damping capacity, sliding quality, and resistance to wear, and of course cost.
Composition and Cooling Rate of Cast Irons:
ADVERTISEMENTS:
Carbon, in cast iron, may be in the combined form as cementite, or in the free form as graphite, or both.
This depends on the chemical composition (including the presence of nuclei of graphite) and the rate of cooling of the casting from the molten state:
1. Composition of Cast Irons:
(a) Carbon:
ADVERTISEMENTS:
As the carbon content increases, the melting point (as compared to steels) is lowered to between 1200° to 1140°C, and thus, carbon acts as a graphitiser. But, more the graphite formed, lower are the mechanical properties.
(b) Silicon (0.5-3.0%):
Silicon mainly controls the form of carbon present in the cast iron. Silicon is a strong graphitiser. Depending on its content (and the rate of cooling), silicon not only helps to precipitate graphite during solidification, but may also graphitise the secondary as well as eutectoid cementite. Once the graphite flake has formed, its shape cannot be changed later by any method. Fig. 15.1 (b) illustrates the effect of carbon and silicon on the structure of white or grey cast irons.
Silicon lowers the eutectic composition approximately by 0.30% carbon for each 1% silicon, i.e. the eutectic composition is then calculated by using CEV. Silicon also lowers eutectoid carbon content. Depending on the silicon content and the rate of cooling, the carbon content of pearlite decreases to be as low as 0.50% with 2.5% silicon.
ADVERTISEMENTS:
Silicon shifts the graphite eutectic line upwards such that the temperature interval between the graphite line and the cementite line increases from 6°C at 0% Si to 35°C at 2% Si (this increases the degree of undercooling to help in graphite formation).
The nature of cast iron, white or grey can be changed by varying both carbon and silicon, and the rate of cooling. For high strengths, carbon is kept on lower side (to have low volume of graphite) and silicon on higher side (keeping a balance to get good machinability). Fig. 15.1 (a) shows that greatest structural strengths is obtained when carbon is about 2.75% and silicon about 1.5%, i.e., when the matrix is completely pearlitic.
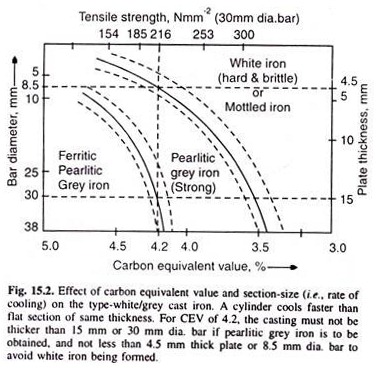
Fig. 15.2 shows that pearlitic grey iron of CEV = 4.2, should be of size between 15 mm to 4.5 mm thick plate, or 30 mm to 8.5 mm dia. bar, illustrating the effect of rate of cooling. Alloying elements, added to impart special properties, all effect the chill. Non-carbide forming elements like Ni, Al, Cu, promote the graphite formation, whereas the carbide forming elements like Mn, Cr, Mo, etc. promote cementite formation.
ADVERTISEMENTS:
Depending on the potency, the affect is normally calculated as silicon equivalent value:
Si Equ. Value- % Si + 3 (% C) + 0.3 (Ni% + % Cu) + 0.5 (% Al) + % P – 0.25 (% Mn) – 0.35 (% Mo) – 1.2 (% Cr) …(15.1)
(c) Sulphur and Manganese:
ADVERTISEMENTS:
Sulphur (0.06-0.12%), when present as FeS (which increases tendency to brittleness) tends to promote cementite formation, i.e., retards graphitisation and increases the size of the flakes. Manganese (0.5-1.0%) is a mild carbide-former and controls the effect of sulphur if enough amount of Mn is present (one part of sulphur to 1.72 parts of manganese), as it has more affinity for sulphur (than Fe has) to form MnS, which rises to the top of casting to join the slag-thus removes the red-shortness of FeS eutectic.
Manganese, thus, has indirect effect to promote graphitisation as it removes sulphur, (which promotes cementite formation). More direct effects of manganese include strong cementite-stabilising effect on eutectoid-graphitisation (around 1% Mn may be added to get pearlitic matrix in graphitic cast irons), hardening of iron, refinement in grains and an increase in strength.
(d) Phosphorus (0.1-0.9%):
When phosphorus is less than 0.3%, it dissolves in ferrite, otherwise, it forms Fe3P which forms eutectic (91.19% Fe, 1.92%C, 6.89%P) called steadite, which is brittle (causes cold-shortness, i.e., castings are unsuitable for shock-resistance) and low melting, M.P. 960°C.
This increases the range of eutectic solidification, and thus, helps to form graphite, and improves castability even of thin and intricate sections. A 1% phosphorus in iron results in steadite which accounts for 10% by volume of the casting; the embrittling effect of steadite is evident.
2. Cooling Rate of Cast Irons:
In Fe-C alloys, although graphite is more stable phase but cementite formation is kinetically favoured as it is easier and quicker (only 6.67% carbon atoms are needed to segregate) to form cementite. A high rate of cooling prevents the formation of graphite at all stages (from liquid to the eutectoid reaction).
However, if silicon content is more than 3%, graphite is obtained even when the casting is rapidly cooled. Fig. 15.2 illustrates the effect of section size, (i.e., the rate of cooling) and the carbon equivalent value on the type of structure, and thus, the type of cast iron obtained.
The presence of inoculants such as Ca, Al, Ti, Zr, SiC, CaSi etc., decreases the size of the flakes and improves the uniformity of their distribution, probably because the nuclei promote nucleation of primary austenite, thus, reducing their grain size, and thus, the size of flakes and better distribution.
Comparison of Properties of Cast Irons:
Table 15.6 compares some properties of some cast irons. Grey iron is the cheapest and the easiest to cast to get sound castings. Compacted graphite irons have superior mechanical properties, even at elevated temperatures than grey iron, but are expensive and are not typically heat-treated.
Meehanite irons is better than grey iron but are a bit expensive. S.G. iron suffers from more shrinkage during casting (requires bigger risers, etc.) and are expensive, but result in much higher strength, ductility and toughness. Malleable irons are difficult to cast (as white iron), and there is restriction about the section-size than S.G. iron.
These generally cost more in the final form than S.G. iron, but thin sections of malleable iron may be preferred for higher toughness; S.G. iron might require annealing for getting more uniform structure.
For distinguishing the irons, S.G. iron gives a definite ring when struck with a hammer (not as clear as of steels) whereas grey iron produces damped sound. However, breathing a freshly polished surface of S.G. iron gives smell of acetylene gas (its magnesium carbide reacts with moisture of the breath).
Science of Development of Microstructures of Cast Irons:
The graphitic cast irons have graphite embedded in steel matrix, i.e., varying proportions of ferrite and pearlite (from zero percent of pearlite to 100%). The properties of the cast irons are determined by the properties both of matrix, and the amount, size, shape and distribution of much needed graphite inclusions (for some properties like machinability, damping capacity, wear resistance, etc.). Graphite flakes in grey iron have weakening and embrittling effect as graphite can be approximated as voids, or sharp cracks breaking the continuity of the ductile matrix.
The sharp-ends of each flake act as an internal notch, which under the stress acts as a stress-raiser to easily propagate the crack in the plastic matrix to give a brittle, sooty, grey fracture at low stresses from 150-400 MNm-2, depending on the nature of the matrix; the maximum value being when the matrix consists of only fine pearlite.
The heat-treatment of grey iron can result in other structures of matrix such as tempered martensite, which normally possesses higher strength properties, but the important properties like tensile strength, toughness, and ductility do not change much, because the flakes cause brittle fracture. The strength properties of grey iron further deteriorate as the volume of the graphite becomes larger, and the flakes become coarser. A closed-network of graphite flakes results in worst mechanical properties.
The increase in strength and toughness can be obtained by making the flakes finer such as in meehanite iron and by reducing the total volume of graphite by having less contents of carbon and silicon. The strength and toughness can then be enhanced by heat treatment, i.e., by changing the matrix.
The embrittling effect of graphite can be drastically reduced as the shape of the graphite changes from flake to spheroidal, as the round graphite inclusions do not produce sharp stress-concentrations as these do not act as sharp cracks in matrix (even the stubby flakes-graphite rods with rounded edges in compacted graphite iron is a lesser stress- concentrator).
Thus, S.G. irons have higher strengths in tension and bending along with ductility. With the same steel-matrix, the ductility (as given by % elongation) of cast irons changes with different shapes of graphite as given i.e., ductility appears to be more dependent on shape and size of graphite than on metallic matrix in graphitic irons.

As nodule form of graphite in S.G. iron (as well as in malleable iron) is not a sharp stress-raiser, and does not act like a crack, a change in microstructure of the matrix by suitable heat-treatment results in appreciable increase in strength properties of S.G. iron (also of malleable iron), and are thus given different heat treatments.
When graphitic cast iron is heated for heat-treatment, it tends to form a protective atmosphere if placed in a tight furnace, or in a box, otherwise, unwanted heavy oxidation occurs. A subscale of iron silicate forms, which can only be removed by molten salt electrolysis (Kolene process). It is better to use protective gas atmosphere, specially for finish-machined parts.
Heating the graphitic iron can modify its matrix. When it is being heated, then at temperatures approaching the lower critical temperature, above about 540°C, its silicon can cause the cementite of pearlite to dissociate into ferrite and carbon. The carbon diffuses to and deposits on the already present graphite.
The lower critical temperature of cast iron is calculated as:
Lower Critical temperature, °C = 730 + 28 (% Si) – 25 (% Mn) …(15.2)
When this cast iron is heated above the critical temperature, the austenite forms and in a short time, becomes saturated with carbon that is dissolved from graphite. The microstructure of the iron at a temperature slightly greater than T2 shall have graphite and austenite of composition point C1 as illustrated in Fig. 15.14.
If the iron is heated to higher temperature, more carbon dissolves from graphite to saturate the austenite at the new temperature. For example, at temperature T (≈ 900°C), austenite has a carbon content of around 1.1% in it. Thus, once the austenite has been obtained, the irons can be given most of the heat-treatments as given to steels, provided commercially economical.
Chemical composition also effects the heat-treatment of cast irons. The unalloyed cast irons have silicon and manganese. Silicon accelerates various reactions taking place during heat- treatment; it decreases solubility of carbon in austenite, increases the diffusion rate of carbon in austenite; raises the austenitising temperature significantly as given in equation 15.2; reduces the volume of cementite in pearlite, i.e., carbon content of pearlite is less than 0.77%, and can be 0.50% with 2.5% silicon.
Manganese has opposite effects- lowers austenitising temperature; increases solubility of carbon in austenite; decreases diffusion of carbon in austenite; increases volume of cementite in pearlite, i.e., increases carbon content of pearlite; stabilises pearlitic carbide, thus increases pearlite content; reduces pearlite spacing, thus induces strength; increases hardenability, but generally decelerates heat-treating reactions.