Various types of point defects are: 1. Vacancy 2. Interstitialcy 3. Frenkel Defect 4. Impurity Atoms.
A point defect is a very localised disruption in the regularity of a lattice. It is a defect of dimensions just like a point (zero dimensions). The size of the defect could be one atom, or two atomic diameters, which is just like a point. A point defect extends its influence only a few atomic diameters beyond its lattice position.
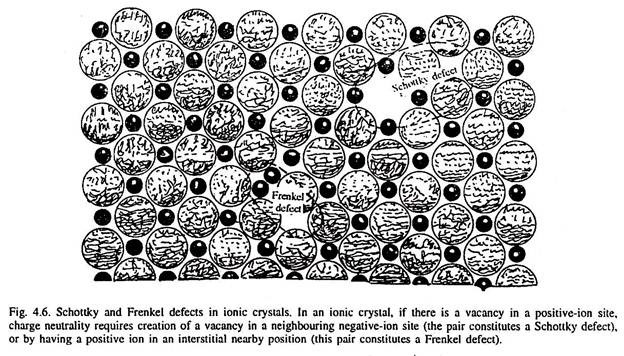
Type # 1. Vacancy (Schottky Defect in Metals):
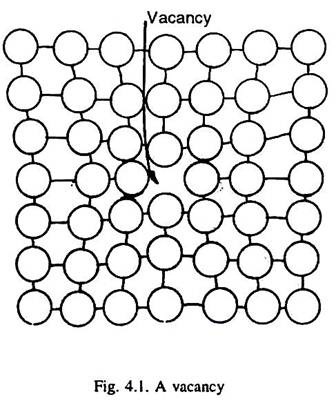
When an atom is missing from its lattice site in a crystal structure of a metal, it is called a vacancy (or vacant lattice site) as illustrated in Fig. 4.1. It may be indicated by a square symbol. The atoms surrounding a vacancy experience a slight displacement into the empty lattice site, and thus, a vacancy is a centre of approximately spherical distortion in the lattice, Fig. 4.1.
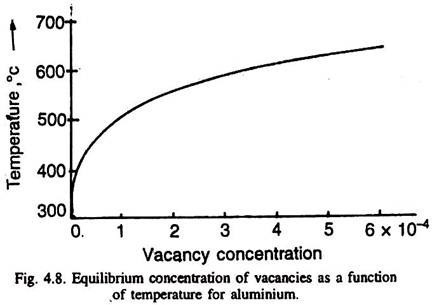
There could be di-vacancies (an association of two vacancies) or even tri-vacancies. A thermodynamic argument shows that vacancies are not only the normal constituent of metals and alloys (above absolute zero degree temperature), but their equilibrium concentration in a metal increases (exponentially) with the increase of temperature Fig. 4.8.
Fig. 4.2. illustrates the schematic steps in creating a vacancy in the crystal lattice. As atom X moves to a kink, rest steps leave a vacancy at point 1. A free surface acts as a source of vacancies (it acts like a sink when required).
Even a grain boundary acts as a source of vacancy as well as its sink. The creation of vacancies becomes easier with the increase of temperature of the metal as thermal energy helps in the diffusion of the atoms. A reverse phenomenon can occur if a given region of a metal is supersaturated with vacancies.
Vacancies play an important role in the diffusion of atoms in common industrial processes like homogenisation, annealing, precipitation hardening, sintering, surface hardening, oxidation and creep of metals. The climb of dislocation is possible because of the presence of vacancies in metals.
The number of vacancies can become more than their equilibrium concentration by one or more of the following reasons:
1. A solid hot metal after fast quenching retains the vacancies.
2. Cold working creates vacancies particularly at jogs.
3. Oxidation of some metals such as Cu, Ni, Zn, etc. causes lattice vacancies.
ADVERTISEMENTS:
4. Vacancies as well as interstitials are created in metals by the bombardment of high energy nuclear particles.
Type # 2. Interstitialcy (Self-Interstitial):
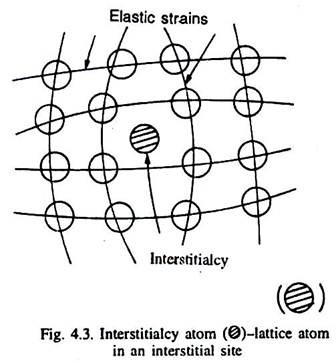
When an atom of the metal occupies an interstitial site (which is not its normal site), it is called interstitialcy as illustrated in Fig. 4.3. Normally, it requires large energy to create an interstitialcy. The size of an interstitial site is very small, and that is why the neighbouring atoms around an interstitialcy, particularly in close-packed crystal structures are much more elastically strained (displaced) than around a vacancy as illustrated in Fig. 4.3.
The number of interstitialcies in an ordinary metal is thus, negligibly small compared to the number of vacancies. Under unusual conditions, such as exposure of the metal to high energy neutrons in a nuclear reactor, or even by drastic cold working, a significant number of interstitialcies can be produced.
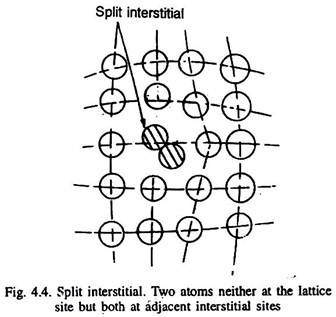
Sometimes, a split interstitial can also form, in which two atoms of the metal are present in two neighbouring interstitial sites with one lattice site vacant as illustrated in Fig. 4.4. Such defects are found in the lattices of low packing factor.
Type # 3. Frenkel Defect:
When an atom is shifted from a normal lattice site (thus creating vacancy) and is forced into interstitial position, the resulting pair of point defects (a vacancy as well as the interstitialcy) together is called a Frenkel defect, as illustrated in Fig. 4.5.
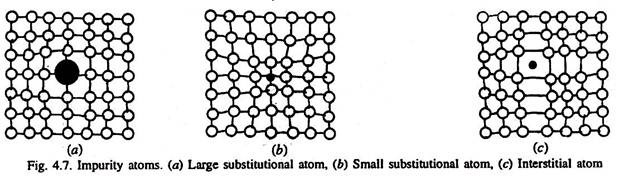
Type # 4. Impurity Atoms (Substitutional or Interstitial Type):
As there are two types of solid solutions, an impurity (outside) atom if present on the lattice by substituting the lattice site atom, then this substitutional impurity atom is a point defect in the crystal structure of pure metal.
ADVERTISEMENTS:
Substitutional atoms may either be larger than the normal atom in the lattice (in which case, the surrounding atoms are compressed, Fig. 4.7 a), or smaller (causing the surrounding atoms to be in tension, Fig. 4.7 b). In either case, the substitutional defect distorts the surrounding lattice. As such a defect can be introduced either as an impurity, or as a deliberate alloying addition, and once introduced, the number of defects is relatively independent of temperature.
An interstitial defect is produced if any of the small sized atoms (of B, C, H, N, O) is present in the interstitial site. The sizes of their atoms are larger than the interstitial site they occupy. Consequently, the surrounding lattice is compressed and distorted, Fig. 4.7 (c). Once these atoms are introduced in the structure (impurity such as hydrogen, or alloying addition such as carbon), the number of interstitial atoms in the structure remains nearly constant, even when the temperature is changed. Normally, the concentration of these atoms in a very pure metal is lower than the concentration of vacancies.
As the defects interact with conduction electrons, their number in a crystal is vital in semi-conductors. Foreign atoms also play a role in changing the electrical conductivity of the semi-conductors.