The presence of alloying elements effects substantially, the tempering behaviour of the steels depending on the nature, amount and simultaneous presence of number of alloying elements. Most common elements (except cobalt) shift the CCT curve to longer times, which essentially result in the increase of hardenability of the steels, so that pearlitic transformation can be avoided easily to obtain martensitic structure, even at slower rate of cooling and in thicker parts.
Alloying elements also lower Ms and Mf temperatures, increasing further the amount of retained austenite. The decomposition of retained austenite on tempering, plays quite a significant role on the properties of tempered steels, specially having high carbon and high alloying elements.
The usual classification of alloying elements can be used here to make general analysis. Some elements, that are not found in carbides, but are present as solid solution in ferrite, are aluminium, copper, silicon, phosphorous, nickel and zirconium.
ADVERTISEMENTS:
Some elements arranged in order of increasing tendency to form carbides are manganese, chromium, tungsten, molybdenum, vanadium and titanium. These carbide forming elements retard most effectively the rate of softening during tempering of the steels.
The first stage of tempering does not appear to be effected by the presence of the alloying elements. However, most of the alloying elements in steels tend to increase the resistance of the steel to softening when tempered, i.e. an alloy steel shows higher hardness after tempering than a plain carbon steel with the same carbon content, though this effect is more significantly shown by alloy steels having appreciable amount of carbide forming elements.
At smaller concentration, they merely retard the tempering processes, hence the softening, particularly at higher temperature ( > 500°C), where these elements have good diffusivity to take part in tempering reactions.
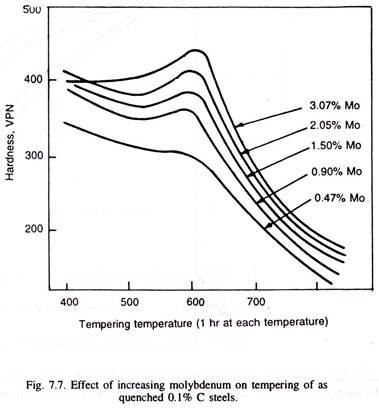
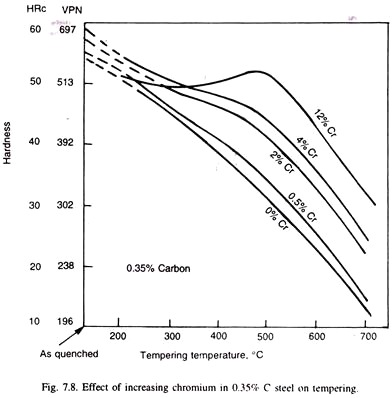
When alloy carbides are formed, the drop in hardness during tempering is not only retarded but is significantly increased. The steel is then said to exhibit the secondary hardening. Thus, 0.5% chromium, or less than 0.5% molybdenum resists softening but secondary hardening is produced by either 12% chromium, or, 2% molybdenum (Fig. 7.7 & 7.8). Stronger the carbide, the more potent is the secondary hardening.
Element, such as, silicon dissolves in ε -carbide to stabilise it. Steels with 1-2% silicon have e- carbide present even after tempering at 400°C, which means that the formation of cementite is delayed considerably, and thus, resisting the softening. Otherwise, the effect of silicon is essentially due to solid solution strengthening. Nickel has a small, but constant effect on tempered hardness at all temperatures due to solid solution strengthening as it is not a carbide former.
Manganese has little effect on softening at low tempering temperatures, but at high temperatures, has a strong effect due to its faster diffusion then, and thus, it resists cementite coarsening as it is present in cementite as (Fe, Mn)3C. Martensite in plain carbon steels loses its tetragonality by 300°C, but the tetragonality is seen at 450°C, or even at 500°C if the steels have elements like chromium, tungsten, molybdenum, titanium, vanadium, silicon.
The basic cause of steep softening in carbon steels on tempering above 400°C, is the coagulation of the cementite particles. Alloying elements notably silicon, chromium, molybdenum, vanadium, when present in steels, retard the coalescence and the coarsening of cementite particles, resulting in enhanced hardening over and above the solid solution hardening effect. They do it either, by entering the cementite structure, or by segregating at the carbide-ferrite interfaces.
The finer cementite particles also resist ferrite grain growth. Elements like chromium, silicon, molybdenum, or tungsten delay coarsening to temperature range of 500-550°C. Up to tempering temperature of 500°C, the carbides formed are of iron with proportional alloying elements in it, but above 500°C, alloying elements can form their own carbides and thus, coarse cementite particles are replaced by fine dispersion of more stable alloy carbides.
ADVERTISEMENTS:
Fig. 7.6 illustrates a good picture of tempering effected by different elements. A Fe-10 Ni alloy shows constant hardness on tempering up to 450°C and then, there takes place some decrease in strength (curve I). Addition of 0.12% carbon increases the as-quenched strength to almost double and slow decrease of hardness occurs on tempering to fall to 0.7 GPa at 500°C. A 8% cobalt addition, which doesn’t enter the carbide, delays the softening to have strength of 0.8 GPa at 500°C.
Addition of 2% chromium almost continuously but slowly increases hardness to start falling at above ~ 450°C to become 1.1 GPa at 500°C by fine dispersion of Cr-carbide. Addition of 1% Mo causes secondary hardening as it is a very strong carbide forming element, to attain a hardness of 1.3 GPa at 500°C.
Secondary Hardening:
In alloy steels, having larger amounts of strong carbide forming elements like Mo, Ti, V, Nb, W, Cr, etc., and carbon, a peculiar phenomenon occurs, the hardness of the as-quenched martensite (called primary hardness) on tempering, decreases initially, as the tempering temperature is raised, but starts increasing again to often become higher than the as-quenched hardness, with much improved toughness, when tempered in the range of 500°C to 600°C, as illustrated in Fig. 7.7.
ADVERTISEMENTS:
This increase in hardness is called secondary hardness (also called red hardness). This is of great importance in high speed steels, as these are able to continue machining at high speeds (as these are able to resist fall in hardness and thus, the cutting property) even when they become red-hot.
Cementite forms and coarsens even in high alloy steels when tempered at low temperatures. The coarse cementite then gets dissolved to give way to more stable alloy carbides, only in the tempering temperature range of 500°-600°C.
This is because, carbon can diffuse easily at lower temperatures to form cementite, as it diffuses interstitially, whereas these carbide forming elements (as well as other metallic elements) diffuse substitutional, and thus, the diffusion of carbon is several orders of magnitude greater in iron than of these elements in iron.
In the tempering range of 500°C-600°C, the carbide forming elements have enough high diffusivity to nucleate and grow to form fine dispersion of alloy carbides to cause secondary hardening. Secondary hardening is a process, similar to age-hardening, in which coarse cementite particles are replaced by a new and much finer alloy carbide dispersion of V4 C3, Mo2C, W2C (which normally form on dislocations).
ADVERTISEMENTS:
As in ageing, a critical dispersion causes a peak in the hardness and strength of the alloy, and as over ageing takes place, i.e., carbide dispersion slowly coarsens, the hardness (strength) decreases. Secondary hardening is best shown in steels containing molybdenum, vanadium, tungsten, titanium, and also in chromium steels at high chromium concentration. The peak is realised at 500°C in chromium steels, 550°C in molybdenum and 550-600°C in vanadium steels and at 600°C in titanium steels.
Fig. 7.7 and 7.8 illustrate effect of individual molybdenum and chromium:
The amount of secondary hardening in an alloy steel is directly proportional to the volume fraction of the alloy carbides, and thus, is directly proportional to the concentration of the strong carbide forming elements present in steel. The alloy carbides must precipitate as fine dispersion and should be uniformly present in ferrite matrix. The massive carbide particles will not contribute much to the secondary hardening.
To obtain the finely dispersed carbides, these carbides must be completely dissolved during austenitisation of the steel, before hardening, to obtain a maximum secondary hardening effect. Fig. 7.9 illustrates the effect of incomplete dissolution of the carbides, because these carbides are quite stable and need very high temperatures of austenitisation. The peak hardness of secondary hardening is low.
Non-carbide forming elements such as nickel, cobalt, silicon do not show secondary hardening. In secondary hardening, additional increment of strength can he obtained by adding less than 0.1% niobium, as the dispersion of NbC in the matrix (not necessarily at dislocations) increases the strength and hardness.
Tempering Characteristics of Alloy Carbide Tool Steels:
The tempering characteristics of the carbide class of alloy tool steels are in some respects different than those of pearlitic class. The carbide class steels show secondary hardening phenomenon, not shown by pearlitic class, and to develop it, the tempering temperatures of carbide class of tool steels is between 500 – 600°C, whereas the pearlitic class tool steels are tempered only in low-temperature tempering range. Another major difference is in the transformation of retained austenite.
In pearlitic class of steels, retained austenite transforms, normally to lower bainite on heating during tempering in range 200° – 300°C (2nd stage of tempering). Whereas, the carbide class of tool steels obtain martensite as the transformation product of retained austenite during cooling from the tempering temperature of 500-600°C (and not on heating).
The curve of the under-hardened steel (austenitised at 1150°C) shows highest as-quenched hardness (of the three austenitising temperatures), due to:
1. Least dissolved carbon and alloying elements in austenite. The presence thus, of undissolved alloy carbides increases the as-quenched hardness of steel.
2. As the austenite has less dissolved carbon and alloying elements, the Ms and Mf temperatures are not lowered much, the amount of the soft retained austenite is less when quenched to room temperature.
3. As the austenitisation temperature is less, grain size is maintained very fine by the presence of undissolved alloy carbide particles, and which produces on quenching a finer and harder martensite.
This under-hardened high speed steel has highest as-quenched hardness, but lacks in the most important property of high speed steels-the red-hardness, which is illustrated by this composition but after austenitising at 1220°C, quenching and tempering at high temperatures (500-600°C).
The high speed steels have to be austenitised at the highest possible temperature without grain coarsening and burning, in order to dissolve as much of alloy carbides as possible, to be finely precipitated during tempering at high temperatures of 500- 600°C. This fine and uniform dispersion causes blockade of dislocations (as in case of age-hardening) to give hardness levels equal to, or higher than as-quenched condition but with higher toughness and wear resistance.
In the as-quenched state, these high speed steels consist of highly alloyed martensite, highly alloyed retained austenite and some undissolved carbide particles. The following flow diagram illustrates schematically the changes on tempering of this state.
The heat treatment of high speed tool steels is different, because of multiple tempering operations given to them. When an as-quenched tool steel having around 20-25% retained austenite is tempered up to 500-600°C there is very little tendency for the retained austenite to transform (which in carbon tool steel transforms to lower bainite during heating in range of 200-300°C) due to deep bay in TIT curves of these steels (i.e., due to high stability of alloyed austenite), but during tempering up to 500-600°C, the retained austenite is said to be ‘conditioned’, for at least some of it transforms to martensite, only on cooling from the tempering temperature.
Probably, during conditioning, austenite becomes more susceptible to martensitic transformation by losing carbon to the existing martensite, i.e., the martensite becomes sink for carbon, because of precipitation of alloy carbides from it. As this diffusion of carbon from retained austenite raises Ms — Mf temperature, a part of it transforms to martensite on cooling.
This martensite which has formed now must be tempered by a second heating to same tempering temperature (500- 600°C). Double tempering is usually sufficient but sometimes as many as four, or even more tempering (same temperature) cycles may be required to bring the retained austenite to an appreciable low level.