In this article we will discuss about:- 1. Introduction to Tempering 2. Aim of Tempering 3. Stages 4. Classification 5. Effects 6. Time and Temperature Relationship 7. Calculation of Hardness of Tempered Steels Based on Composition 8. Industrial Practice.
Contents:
- Introduction to Tempering
- Aim of Tempering
- Stages of Tempering
- Classification of Tempering
- Effects of Tempering
- Time and Temperature Relationship in Tempering
- Calculation of Hardness of Tempered Steels Based on Composition
- Industrial Practice for Tempering
1. Introduction to Tempering:
Martensite, normally, is very hard and strong, but it is very brittle too, and thus, the as-quenched steels find very few engineering applications. Moreover, such a steel, if used even at room temperature, may develop distortion and cracks due to stresses induced in steels during quenching, and also the metastable martensite and the retained austenite may slowly decompose (the former to tempered martensite and latter to martensite) even at room temperature, to cause dimensional changes (as a function of time) due to differences in specific volumes of the parent and the product phases, which may create stresses in adjacent structures in machines.
ADVERTISEMENTS:
But the properties of martensite (or as-quenched-state structure) could be modified, as it is a supersaturated solid solution of carbon in iron, and rejects, on heating, carbon in the form of finely divided carbide phase. As a result, some decrease in hardness occurs, but there is a progressive increase in ductility and impact strength. Thus, the formation of martensite provides a basis to obtain a very wide range of combination of mechanical properties. To obtain these, a heat treatment called tempering is done.
Tempering is the process of heating the hardened steel to a temperature maximum up to lower critical temperature (A1), soaking at this temperature, and then cooling, normally very slowly.
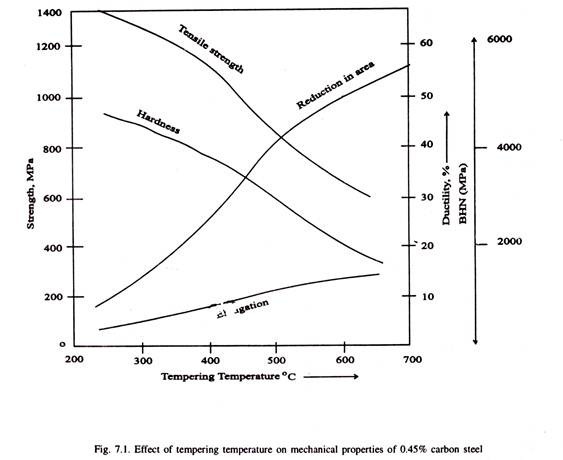
The tempering temperature is decided by the strength (or hardness) and toughness required in service for a given application. Table 7.1 illustrates tempering temperatures of some plain carbon tools. In general, martensite decomposes on heating during tempering, resulting in decrease of hardness and strength but improvement in ductility and impact strength as illustrated in Fig. 7.1.
2. Aims of Tempering
:
1. To relieve internal stresses developed during hardening.
2. To restore ductility and toughness at the cost of hardness and strength.
3. To improve dimensional stability by the decomposition of retained austenite.
ADVERTISEMENTS:
4. To improve magnetic properties by transforming non-magnetic austenite to magnetic product.
Structure in as Quenched State:
The as-quenched steel has a complex structure consisting normally of:
1. Highly Supersaturated Martensite:
ADVERTISEMENTS:
Lath, or plate type, the former having high dislocation density of around 1012cm/cm3 the latter with lesser dislocation density, but may be heavily twinned as the carbon content of the steel increases.
2. Retained austenite, whose amount depends on carbon and alloying elements (also the ambient temperature). Plain carbon steels having carbon below 0.5%, have retained austenite below 2%, is 6% at 0.77%C, but over 30% at 1.25% C.
3. Undissolved Carbides:
Such as proeutectoid cementite in hypereutectoid steels, or, say vanadium carbide in high speed steel (18/4/1) to control grain size.
ADVERTISEMENTS:
4. Rods, or plates of carbide particles produced during ‘auto-tempering’. In steels, having high Ms temperatures, the initially formed martensite gets tempered (still at higher temperatures) during the remainder of the quench to room temperature.
5. Segregation of carbon:
Segregation of carbon to low energy sites such as to dislocations, or vacancies, or as clusters along (100) planes in lath martensite, or along (112) twinning planes in plate martensites with twins, takes place. Segregation can occur during quenching between Ms and room temperature, or at room temperature during holding, or even during heating to about 100°C during tempering. At about 0.2% carbon in steel, defect sites become almost saturated with carbon, and the remaining carbon (if present) in steels remains in normal interstitial sites. Actually, 0.20% carbon is also the point at which tetragonality of martensite can be detected, i.e. martensite is BCC if the carbon of the steels is up to 0.20%, otherwise, it is BCT.
3. Stages of Tempering:
During heating for tempering, intention is to allow the diffusion processes, the nature of which depends on the temperature of tempering.
Tempering of carbon steels takes place in four distinct but overlapping stages:
1. First Stage of Tempering:
Up to 200°C- Precipitation of e (epsilon)-carbide due to decrease of tetragonality of martensite.
2. Second Stage of Tempering:
200° to 300°C- Decomposition of retained-austenite.
3. Third Stage of Tempering:
200° to 350°C- Formation of rods, or plates of cementite with compete loss of tetragonality of martensite and dissolution of e-carbide.
4. Fourth Stage of Tempering:
350°C to 700°C- Coarsening and spheroidisation of cementite along with recovery and recrystallisation of ferrite.
4. Classification of Tempering:
Tempering, in general, has been classified in three categories depending on the tempering temperature range, which depends on the properties to be developed in the hardened steel. Tempering relieves completely, or partly internal stresses developed during quenching-such as, these are more completely removed at higher temperatures, say by a time of 1.5 hours at 550°C.
1. Low Temperature Tempering (1-2 Hours at a Temperature up to 250°C):
Low temperature tempering is done to reduce brittleness without losing much hardness. The tempered martensitic double-phase structure increases the strength with some improvement in toughness, and reduction in internal stresses. Tempered plain carbon steels (0.6 to 1.3% C) have a hardness of Rc 58 to 63. This treatment is given normally to tools of plain carbon and low alloy steels, where the main properties to be developed are high cutting-ability, wear and abrasion resistance with some toughness.
Increasing tempering temperature in this range reduces the hardness slightly but increases toughness with more relief from internal stresses. Low temperature tempering is done either in oil baths (up to 250°C-silicone oil), or in salt bath, or in an air-circulated furnace (as below 500°C, heat transmission through air is very slow). Low temperature tempering is also applied to components, which undergo surface hardening treatments and case hardening treatments, like carburising, cyaniding, or carbonitriding.
Temper Colours:
In earlier times and, at times now too, the tempering temperature attained by plain carbon and low alloy steel component is determined, by the superficial colours developed on the colour scale is called ‘temper colours’. These develop on the clean steel surface as the temperature is raised above 220°C.
The surface of the steel should he ground clean to judge the tempering temperature by the colour of the surface, when heated in a muffle-furnace. Salt bath tempering temperature can be decided by the clearly visible colour, when steel attains the bath temperature.
Temper-colours develop due to the formation of an extremely thin, transparent (initially) film of iron- oxide. Interference of light occurs in this thin surface Film, which appears as temper colours depending on the thickness of the film. This method of determining tempering temperatures by colours is based on the fact that each temperature pertains to a certain thickness of the oxide film, which in turn gives a certain colour.
The particular temper colour corresponds to a particular above-mentioned temperature only at the instant of its formation, or when held for only a short period of time (up to 2-3 minutes), and if the given temperature is maintained for longer duration of time, the thickness of the film shall increase to change into a colour characteristic of higher temperature. This is true, as temperature and time both are effective in changing the tempering behaviour as well as thickness of oxide film. As the temperature becomes higher than 325°C, the oxide film becomes opaque to become grey.
2. Medium Temperature Tempering (350 C to 500°C):
This range of tempering produces ‘troostile’ microstructure indicating development of high elastic limit with good toughness and hardness in range of HRC 40-50. Endurance limit can be increased by water-quenching the component after tempering in range of 400-450°C which induces compressive stresses in the surface layers. Because of high elastic limit and endurance limit, the range is mainly used for springs of both types, coil and laminated, and also for dies. Care must be exercised to avoid 350°C embrittlement.
3. High Temperature Tempering (500-650°C):
Higher is the tempering temperature of plain carbon as well as low-alloy steels, higher is the toughness developed. This range of tempering produces ‘sorbitic’ structure in steels which, induces best combination of strength and toughness for machine components. Structural steels having carbon 0.3-0.5% are commonly given high temperature tempering. Such a treatment for 1-2 hours is almost able to relieve completely the residual-stresses developed during quenching.
5. Effects of Tempering:
i. Effect of Tempering of Steels on Carbon:
Carbon plays a very significant role on the behaviour of steels during tempering. The as-quenched hardness of martensite is mainly dependent on the carbon content of the steels (Figs. 7.4 and 7.5), and so also the morphology of martensite from lath type to heavily twinned plates. Ms and Mf temperatures are lowered as the carbon content of the steel increases, i.e., it reduces the chances of auto-tempering, and also increases the amount of retained austenite.
The formation of e-carbide is missing in the first stage of tempering if the carbon in the steel is below 0.2%, infact, in such steels, martensite is BCC. Both Fig. 7.4 and 7.5 show effect of tempering temperature for a constant tempering time of 1 hour at each tempering temperature.
ii. Effects of Tempering of Steels on Mechanical Properties:
In the first stage of tempering of steels having carbon more than 0.2%, martensite decreases its tetragonality, which decreases the hardness of the steels, but there also takes place precipitation of ε-carbide, which increases the hardness of steels, proportional to its amount formed. Thus, up to a tempering temperature of 200°C, depending on the net result of these two effects, the hardness of steel normally decreases continuously but only slightly.
In fact, in high carbon steels, having carbon of say 1.2% (Fig. 7.5), a slight increase in hardness is observed in this range up to 200°C, due to relatively high volume fraction of e-carbide formed, which not only compensates for the loss of hardness due to decrease of tetragonality, but gives a small overall increase of hardness. Such a steel also shows a similar hump in the curve (Fig. 7.5) in the temperature range of 200°-300°C, when the increased amount of soft retained- austenite transforms to more hard lower-bainite.
On tempering, in the third stage, there is a marked softening due to sharp decrease in hardness, due to dissolution of ε -carbide in the matrix and the complete loss of tetragonality of martensite, although the precipitation of cementite at this stage does contribute to some increase of hardness, but the overall effect is of softening. At this stage, the steel consists of ferrite and small cementite particles.
Further softening occurs due to the growth in size and the decrease in the number of cementite particles as temperature rises to become closer to A1. The phenomenon of recrystallisation of ferrite, occurring above 600°C, also reduces the dislocation density.
6. Time and Temperature Relationship in Tempering:
The tempering changes in hardness as a function of tempering temperature, where tempering time is kept constant of 1 hour at each temperature. For a given steel, a heat treater might like to choose some convenient time, Say over night, or otherwise different than 1 hour, and thus, wants to calculate the exact temperature required to achieve the same constant hardness.
Hollomon and Jaffe’s “tempering parameter” may be used for this purpose as it relates the hardness, tempering temperature and tempering time.
For a thermally activated process, the usual rate equation is:
where, t is the time of tempering to develop a given hardness, and Q is the ’empirical activation energy.’ ‘Q’ is not constant in the complex tempering process but varies with hardness.
Thus, hardness was assumed to be a function of time and temperature:
Interestingly, [t e-Q/RT] is a constant, and let it be t0. Equating the activation energies of equation 7.1 and 7.2, i.e.
As t0 is a constant, then-
H = f[T(C + In t)]….(7.4)
where, C is a constant, whose value depends on the composition of austenite. The single parameter which expresses two variables time and the temperature, i.e., T (C + In t] is called the Hollomon and Jaffe tempering parameter. To obtain good results, the hardness should be measured on Vickers Scale.
The exact value of C though is not critical, but with temperatures in °F, and tempering time in hour, a value of 18 gives better results. Suppose for a given hardness value, t and T are known, then, say T(C + In t) gives a value of 30,000. Now a convenient time can be chosen, and putting that in the parameter, find the temperature, which gives a value of 30,000 of the parameter. Computer could be used to get this result.
Fig. 7.10. illustrates a graph which could be used to convert one tempering temperature and time to another tempering temperature and time, on the basis that combinations of tempering temperature and time having the same value of the tempering parameter, produces the same hardness. For example, 50 hrs at 900°F or 2 hrs at 1000°F or 7 minutes at 1100°F should produce the same hardness.
Here, 50 hours may be too long a time, and 7 minutes may be too small a time for tempering and thus, 2 hours at 1000°F appears to be a convenient time.
Though tempering parameter has been used usefully for the plain carbon and low alloy steels, but much more caution must be used in applying it to secondary hardening steels (it has been used successfully there too). For example, a higher maximum hardness can be obtained on tempering at 600°C rather than at 700°C, and it will be impossible to reproduce the 600°C hardness maxima even with a very short time of tempering at 700°C.
7. Calculation of Hardness of Tempered Steels Based on Composition:
Grange’s method could be used to calculate the hardness of the tempered martensite in carbon and low alloy steels. The chart in Fig, 7.11 is used to calculate the hardness of the Fe-C base composition i.e. based on carbon in steel and the tempering temperature. It is assumed that hardening effect of each alloying element is the same at all the carbon contents in steels.
Thus, for the fixed tempering temperature, for which hardness was read from the Fig. 7.11 for carbon, addition is made to this, the AH VPN values of each of the alloying elements present in steel for that tempering temperature.
Calculated VPN = ΔH VPNC + ΔH VPNMn + ΔH VPNNi + ΔH VPNCr + …
Equations for various curves could be framed for the effect of an element at different tempering temperatures. This could be done for other elements. Now, the computer could be used to calculate the hardness to be obtained after tempering a steel of known composition at a temperature.
This hardness compares well with actual results with 5 to 10% variation. Computer could be used to finalize the composition of the steel to get a desired hardness after tempering at a particular temperature.
8. Industrial Practice for Tempering:
It is a rule that a steel which has been hardened, must be tempered, but it is more important that it is tempered as soon as possible after quench-hardening. History of tempering practice is full of reported cases, where tools, especially those of intricate shapes, which had been satisfactorily quenched without any cracks during cooling, suddenly develop cracks while lying undisturbed on stands. Some cracked pieces of quenched rolling rolls flew quite a few meters, when lying unstressed.
This is due to severe internal Stresses set-up by quenching. Thus, such tools should be preferably put into a tempering furnace immediately, when the quenched part has been cooled to between 75° to 50°C. Martensite that forms between 75°C and room temperature may induce cracking, or some isothermal martensite may form when part is held at room temperature.
The temperature of interruption does depend on Ms – Mf temperatures, which are dependent on the alloy content of steels. In practical terms, the part is allowed to be cooled during quenching to the state that it can just be held in hands (although, the temperatures inside the section of the part are higher), and then immediately transferred to the tempering furnace. Such a practice does not induce uniform properties across the section of the part.
Heating of the part for tempering should be done slowly. Fast heating, such as, in agitated salt baths, causes surface layers to increase in volume, which can induce unfavourable stress conditions to produce cracks. (Hardened parts austenitised in salt baths should be freed from any adhering salt before tempering is done, by rinsing the parts in warm water, otherwise salt residues cause corrosion during tempering, unless the tempering is to be also done in a salt bath (nitrate-nitrite type) in which the hardening salt gets dissolved.
Tempering salts are normally soluble in warm water). The time of tempering is normally 1-2 hours per 2.5 cm of section thickness. The time is counted the moment the furnace has reached preset temperature, or right when the charge is put in the furnace, if the furnace is at the preset temperature of tempering.