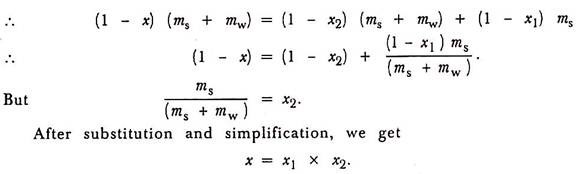There are four methods of determining the dryness fraction of steam.
They are as mentioned below:
1. Bucket Calorimeter:
ADVERTISEMENTS:
This method of determining the dryness fraction of steam is not exact. The value of dryness fraction obtained by this method is lower than the actual value.
The apparatus shown in fig. 3-21 consists of a copper calorimeter which is placed on wooden blocks in a vessel. The vessel is large enough to provide an air space round the calorimeter so as to provide insulation to prevent heat loss as far as possible. The top cover is made of wood and it closes both the calorimeter and the vessel.
The cover has two holes. Through one hole the steam pipe is led into the calorimeter. The steam is distributed in the water in the calorimeter by the holes in the bottom ring which is connected to the end of the steam pipe. A thermometer is inserted from the second hole to measure the temperature of water in the calorimeter.
Before starting the experiment the copper calorimeter is weighed and by knowing the specific heat of copper the water equivalent of the calorimeter is known. Then necessary amount of water is taken, in the calorimeter and its initial temperature is noted. The calorimeter is placed in the vessel and the top cover is placed in position and steam connection to steam pipe is made.
ADVERTISEMENTS:
The steam is passed through the water. The steam comes in contact with the water. It condenses and gives out its entire latent heat and part of its sensible heat. Due to this heat transfer the temperature and mass of the water in the calorimeter increases by the amount of steam condensed.
Sufficient quantity of steam should be blown in the calorimeter so that there is a sufficient rise in the temperature and thereby errors are reduced to minimum. Afterwards the steam cock is closed. While passing the steam through water in the calorimeter care should be taken in opening the cock.
The cock should be opened by such an amount that all the steam gets condensed in water and no steam bubbles escape out of water. If the bubbles escape then the error may be introduced in calculation.
ADVERTISEMENTS:
The steam pipe is disconnected and the cover is removed and the mass of mixture is determined. The increase in the mass of the water is the mass of the steam condensed.
Let P = the pressure of steam in a steam pipe
hf1 = sensible heat corresponding to pressure P
t2 = temperature of water and vessel before experiment
ADVERTISEMENTS:
t3 = temperature of water and vessel after experiment
h2 = liquid heat corresponding to t3
hfg1= liquid heat corresponding to t3
hfg1 = latent heat of steam corresponding to p
ADVERTISEMENTS:
x = dryness fraction of steam in the steam pipe
mw = mass of water in the calorimeter
mc = water equivalent of calorimeter
ms = amount of steam condensed during the experiment
Cpw = specific heat of water.
Heat given out by steam = Heat received by water and the calorimeter.
Qs = Qwc
Heat given out by steam Qs = ms (hf1 – h3 + xhfg1).
Heat received by water and calorimeter
mwc = (mc + mc) x (h3 – h2) x Cpw
∴ We get ms (hf1 – h3 + xhfg1) = (mc + mw) x (h3 – h2) x Cpw.
From the equation the value of x can be found out.
In the calculation we assume that no heat is lost to the surroundings and the steam enters the calorimeter as vapour and not as condensate.
2. Separating Calorimeter:
This is shown in fig. 3-22. It consists of a .device for trapping the water and draining it off. The steam which escapes the instrument is condensed. While calculating the dryness fraction of the steam it is assumed that all the water particles in suspension are separated out and the steam leaving the separator; is dry. This assumption is not correct so the results obtained are approximate. This calorimeter is used with throttling calorimeter in practice.
Let ms be the mass of water obtained from the condenser i.e., the amount of steam condensed during the test.
Let mw be the mass of water obtained from the separator and x be the apparent dryness fraction of the steam sample.
3. Throttling Calorimeter:
This is shown in fig. 3.23.
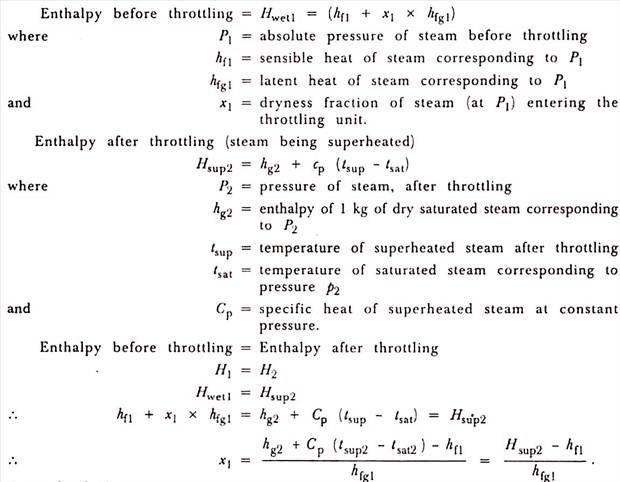
When steam is allowed to pass through a restricted opening such as a partially closed valve, then the steam is throttled. The pressure of steam decreases but the volume increases. If no heat is lost by leakage to atmosphere, the total energy of the steam is the same before throttling and after throttling.
Since the total heat of steam remains the same after throttling, the steam becomes drier. If the dryness fraction of steam is high initially, then after throttling the steam gets superheated and the temperature of superheat can be measured by a thermometer. The pressure of the steam after throttling can be read by a manometer.
As the pressure and the temperature of the steam after throttling are known, the enthalpy of steam after throttling can be calculated. Since the enthalpy before throttling is equal to enthalpy after throttling, this allows us to calculate unknown quantity which is the dryness fraction of steam before throttling.
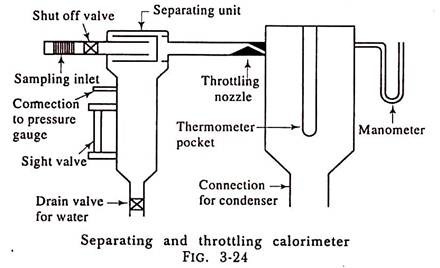
4. Combined Separating and Throttling Calorimeter:
The separating calorimeter when used alone does not give a very accurate result because we have to assume that all the water is separated out from the steam while passing through the calorimeter, which is not the case. Also the throttling calorimeter is only able to measure the dryness fraction of steam if the Steam gets superheated after throttling.
If the initial dryness fraction of steam is sufficiently low, the steam will not get superheated after throttling and the dryness fraction of the steam cannot be found out with the help of throttling calorimeter alone. In such a case the dryness fraction x of steam can be found out by using the separating and throttling calorimeter combined.
The separating unit mechanically collects most of the moisture from the steam sample passing through it and allows comparatively dry steam to flow to the throttling unit where superheating takes place without change of enthalpy.
Fig. 3-24 shows the combined separating and throttling unit.