In this article we will discuss about:- 1. Introduction to Carburising of Steels 2. Structure and Properties 3. Mechanism of Carburising of Steels 4. Theory 5. Depth 6. Plasma Carburising 7. Carburising Furnaces.
Contents:
- Introduction to Carburising of Steels
- Structure and Properties of Carburised Steels
- Mechanism of Carburising of Steels
- Theory of Carburising of Steels
- Depth for Carburisation of Steels
- Plasma Carburising of Steels
- Types of Carburising Furnaces
1. Introduction to Carburising of Steels:
It is the process of enrichment of a surface depth of low carbon steels with carbon. It is then hardened, and is thus, also called case-hardening. It leads to the development of a combination of high surface hardness as well as high toughness and impact strength due to core, as required for many engineering parts in service life such as heavy duty gears, cams, ball bearings, bushings, rock-drilling bits, etc.
ADVERTISEMENTS:
The base steel, the low carbon steel (0.1 to 0.25%C) as a rule is readily machinable, to easily produce different shapes, as well as impart good toughness and impact strength to the components. The carbon- enriched-surface-depth has compressive stresses developed in it (due to presence of carbon) to impart good fatigue strength to the component. This surface-depth can be hardened to develop high hardness and wear resistance.
Carburising is achieved by keeping the low carbon steel in contact with solid, liquid or gaseous atmosphere of high carbon activity at high temperatures, i.e., in the austenitic range. Carbon atoms liberated from the surrounding medium are absorbed at the steel surface, which then diffuse inwards, enriching the surface with carbon.
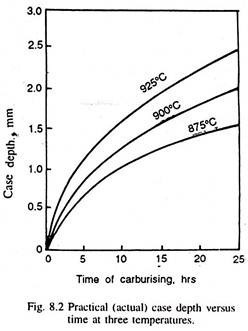
The carbon content of the surface is controlled by the activity of the carbon in the surrounding medium (called carbon potential of the atmosphere), or the maximum solubility of carbon in austenite at the carburising temperature, whichever is less, but the depth of penetration of carbon is dependent on the temperature and time of the carburising (Fig. 8.2). The enriched surface layer is generally called case, and the central remaining part is called the core.
2. Structure and Properties of Carburised Steels:
ADVERTISEMENTS:
The aim of carburising is to get a case of high carbon martensite with resultant good wear resistance and fatigue strength and a tough low carbon core. The core of carburised plain carbon steel having carbon 0.1-0.25% has low hardenability and consists of ferrite and small amount of pearlite.
In quite a few heavy duty applications, the core is required to have high strength, for example, to:
ADVERTISEMENTS:
(i) Support the strong case.
(ii) Have less stress gradient between the case and core otherwise subsurface cracks can nucleate in core.
Thus, for such applications, alloy steels are used to have good core hardenability so that martensite forms throughout the part.
Carburised steel, after diffusion step and quenching containing around 1%C. Plate martensite with 20% or so of retained-austenite is seen. This is a common practical microstructure.
ADVERTISEMENTS:
Gas-carburising often shows oxidation of grain boundaries to a depth of 0.025 mm from surface. This is due to the oxygen in the carburising atmosphere. Elements like Cr. Mn, Si, Ti intensify oxidation.
The removal of silicon eliminates the surface oxidation. This intergranular oxidation is detrimental to fatigue strength, and thus, in critical parts, grinding or machining of this layer is done or vacuum or plasma carburising may be used, not to have it at all in carburised case.
High surface carbon leads to plate martensite with large amount of retained austenite. Plate impingement may cause micro-cracking, which can be reduced if austenite is fine and when the lath martensite forms due to lower carbon in martensite. This also helps in reducing the fatigue failures.
3. Mechanism of Carburising of Steels:
ADVERTISEMENTS:
When a low carbon steel is brought in intimate contact at the austenitic temperature range with solid, liquid or gaseous carburising medium, which liberates free carbon by means of chemical reactions (which might be catalyzed by iron), carburising occurs.
The process of carburising takes place in two steps:
In the first-step, the free-carbon is added i.e. absorption of carbon takes place at first rapidly at the surface of the steel because there is large difference between carbon potential of the atmosphere and the carbon content of the steel surface. As the surface carbon content increases, the rate at which additional carbon content can be absorbed decreases, till it becomes equal to that of the atmosphere.
In the second-step, carbon of the surface diffuses inside the case. In the beginning, this diffusion rate is small as the carbon gradient (and thus, the driving force for diffusion) is small between the surface and the core. This concentration gradient of carbon increases as more free carbon is absorbed by the surface.
It has been seen that the first step is of relatively short duration as compared to the second step, i.e., the maximum carbon content at the surface is attained in a shorter time, and is then maintained at this value for the latter process. The depth of carburisation is dependent on the diffusion of carbon from the surface to the interior.
4. Theory of Carburising of Steels:
The steels used for carburising usually contain carbon 0.1 to 0.25%. Carburising is carried out to obtain a surface carbon content of 0.6 to 1.10%. With still higher surface carbon content, the case becomes very brittle due to coarse cementite network formed in it.
Carburising is done in the austenitic range (because the solid solubility of carbon in austenite is much higher than in ferrite, and which increases with the rise of temperature), usually between 850°C to 950°C, but commonly at 900 to 925°C mainly not to decrease the life of furnace parts.
Surface Carbon Content:
Generally, the specifications of carburised steels are specified by (i) minimum surface hardness, (normally Rc 50), or (ii) a case-depth of specified range. The surface-hardness depends mainly on the surface carbon-content.
When low carbon steel at high temperature is in contact with an atmosphere of high carbon activity, carbon is absorbed at the surface. The carbon content at the surface increases till the carbon activity at the surface of the steel equals the carbon activity in the atmosphere.
However, if the activity of the carbon of the atmosphere is higher than the solubility limit of the carbon in the austenite at the carburising temperature, then the carbon content of the surface cannot be increased beyond the solubility limit.
These solubility limits is given by the Acm line in Fe-Fe3C diagram for Fe-C alloys and are:
Fig. 8.3 illustrates it and solid solubility limit for some alloy steels. Thus, the maximum surface carbon content is limited by the carbon potential of the atmosphere, or the solubility limit of carbon in austenite at the carburising temperature, whichever is lower.
Normally, the desired surface carbon content is lower than the solubility limit, because having carbon content beyond 0.8% doesn’t increase the hardness of martensite obtained on hardening, as more and more retained austenite is obtained, which is quite undesirable, as it leads to lower hardness values and is a potential source of dimensional and microstructural instability.
Thus, commonly, the carbon potential of the atmosphere is controlled to achieve desired carbon content at the steel surface. The main advantage of using higher carburising temperatures is to increase the absorption and the diffusion rates to reduce the carburising times.
5. Depth for Carburisation of Steels
:
It is defined as the perpendicular distance in mm from the surface to a plane at which the hardness is HRc 50 or 550 VPN (≈ 0.4% carbon). This is also called effective case depth. Sometimes, it is taken to be the distance in mm from the surface, where the hardness specified in HRc is lower by 15% than the hardness specified for the surface of the component. (i.e. 60-60 x 1.5 = 51 HRc)
Fig. 8.7 Hardness profit of case in case-handed steel DC is 1 mm.
Fig. 8.7 illustrates a general hardness profile of case-hardened steel with clear distinction between effective case depth and total case depth. More quick, simple and approximate method used in; practice consists of withdrawing a part or sample from furnace, quenching in water and fracturing with hammer.
The fracture surface is examined at 10 X with a magnifying lens to approximately assess the case depth. Fig. 8.8 (a) illustrates a free cutting steel. Carburised and quenched from 850°C showing dull grey colour case with coarse grained core as schematically illustrated in (b).
This depth up to HRc 50 (DC) depends on:
(a) Carbon content of case
(b) Section size particularly when these are thick. This does not apply to gears as rate of cooling of gear tooth is greater than of a solid shaft,
(c) Coolants- Water quenching gives greater depths particularly in thick sections,
(d) Grade of steel- More highly alloyed steel gives greater DC for thick parts. Due to increased hardenability of more highly alloyed steels, carburising time is shorter
(e) Quenching temperature- If hardenability is effected by quenching temperature, it also effects the depth temperature, the latter also decreases.
Evaluation of the Depth of Carburisation through Fick’s Law:
The depth of carburisation is the distance below the surface to definite carbon content, or the total depth of carbon penetration. It depends on the time and temperature of carburising, the carbon potential of the medium and the composition of the steel. The higher the carbon potential, higher is the carbon concentration at the steel surface, and deeper is the carburising depth. (Fig. 8.4).
The depth of carburising as a function of time can be evaluated by using Fick’s laws of diffusion for semi-infinite case of infinite source. It can be safely assumed that the surface carbon content reaches the maximum value, Cs instantly and remains constant at this value during carburising. The carbon content of the interior equals the original carbon content of the steel, C0.
A simple approximate solution can be arrived at:
Fig. 8.5 (a) illustrates the start of carburising when surface has attained the maximum carbon content from the atmosphere to become Cs. After a time (t > 0) of carburising, the variation of carbon concentration may be taken to vary linearly from Cs at the surface (x = 0) to C0 at a distance x, the carbon content equal to the original carbon content of the steel, where x is the case depth (Fig. 8.5 b).
As carburising proceeds, the amount of carbon atoms added per sec to the steel is given by Fick’s first law as:
where, J is the net carbon flux per unit area per second, Dcy is the diffusion coefficient of carbon in austenite. If it is assumed that the case depth increases from x to x + dx as the carburising time increases from t to t + dt. To increase the case depth from x to x + dx, the amount of carbon as illustrated by the hatched area in Fig. 8.5 (b) must be added to the steel. This amount of carbon is supplied by the diffusion of carbon from the surface of steel in time, dt, which is equal to J.dt, and thus-
The right side of the equation (8.4) gives the hatched area of Fig. 8.5 (b). Putting the value of J from equation (8.3) to equation (8.4), and rearranging gives,
This is Harris equation, which has proved adequate for plain carbon and alloy steels. The parameter ɸ as a function of temperature and includes the temperature dependence of the diffusion coefficient. This equation is used to calculate the total case depth. The value of ɸ for different temperatures is given below. Here x is taken in mm and t, time in hours. Equation (8.7) is an approximate solution of the diffusion problem.
Here also, it is taken as a semi-infinite solid with the assumption that instant and constant surface carbon content of Cs is obtained. This differential equation (8.8) has been solved to give carbon content as a function of time and distance as
where, Cx is the concentration of carbon at any distance x from the surface. The boundary conditions are satisfied. Here Dyc does vary with concentration, but making it constant gives simple solution to the equation which is quite applicable to practical problems.
Fig. 8.5 (c) illustrates the curved line for actual carbon distribution in a carburised low carbon steel. This curve is asymptotic to the horizontal line representing the original carbon content of the steel [after carburising, it is the carbon of the core).
According to this curve as well as equation (8.9), Cx = C0 only a x = ∝ but it appears to be very close to C0 at a certain distance from the surface. Now a straight line may be drawn through the surface carbon content such that the areas under both the curves are equal.
Then the distance at which such a straight line intersects the C0 line is defined as the total case depth, or depth of carburisation. It has been seen that case depth determined like this agrees well with the measurements done by metallographic test and represents normally a point about 0.04% carbon higher than the core carbon content.
The equations 8.7 and 8.9 may not be strictly valid for the curved surfaces as these equations have been obtained for planar steel surfaces, (when carbon diffuses perpendicular to the surface), however for most practical carburising problems, it has been seen that these equations give results even with curved surfaces with not much error.
One caution has to be observed while using these equations, is that when heavy cold charge is added to the carburising furnace, it lowers the temperature, and substantial time is taken to reach the carburising temperatures and not much carbon diffusion takes place during this time as the temperatures are low. These equations, then act as a guide and the actual time must be obtained for the case depth for actual operating conditions.
The presence of alloying elements has strong effects on the structure of the carburised layer, mechanism of its formation and the rate of diffusion. Carbide forming elements if present, may cause the formation of two-phase layer by the carburising, consisting of austenite and globule shaped alloy carbides, and thus, the total carbon concentration at the surface may even exceed the solubility of carbon in austenite at that carburising temperature, i.e., steels having elements like Mn, Cr, Mo, W, V may have a total carbon content of 1.0 to 2.00%.
An alloying element may not have the same effect on two steps during carburising, i.e. on the surface carbon content, and the diffusion coefficient. The alloying element, thus, effects the case depth depending on the predominating of these two factors. Cr and W reduce the diffusion coefficient, Dcy of carbon in austenite as this increase the activation energy, but increase the surface carbon content, and thus increase the case-depth to some extent. Ni increases DcY but reduces the surface carbon content, and thus, reduces case-depth. Mn increases surface carbon content but almost has no effect on Dcy, and thus increases the case-depth.
As the hardenability of plain low carbon steels after carburising is low, the core has relatively low strength as it consists mainly of ferrite and some pearlite. Applications which require high core strength to support the case in heavy duty conditions or, where the stress-gradients between the surface and the interior of a part in service are high to produce sub-surface cracks in un-hardened core, alloy steels are used with good core hardenability that form martensite throughout a carburised part.
Thus, the properties of core also form the basis of mechanical properties while designing heavy duty gears etc. Sometimes, the core may have high hardness (may be the carbon is slightly more).
This high hardness, in light sections particularly, just below the carburised layer may give machining problem when the core is being machined after hardening treatment. In such cases, core hardness can be decreased by decreasing the quenching temperature. For example a core with 0.15% C has 50% ferrite when quenched from 780°C, but 5% ferrite if quenched from 830°C with hardness difference of 60 VPN between them.
6. Plasma Carbursing of Steels:
Plasma carburising is basically a vacuum process in which carbon is imparted to the surface of the steel as carbon-bearing ions escaping from an ionised gas or plasma for subsequent diffusion below the surface. A plasma is an electrically generated ‘gaseous’ mixture consisting of positively and negatively charged particles as well as neutral species.
The plasma utilised for case-carburising is the glow discharge having high density of electrons 1 x 1012/cm2 with an average energy in the range of 1 to 10 eV, which can effectively ionize and dissociate diatomic molecules. Thus, in glow-discharge plasma, active carbon for adsorption is formed directly from methane owing to ionising effect of the plasma, and partial heating of parts also occurs.
Plasma carburising is done in oxygen-free atmosphere which permits higher temperatures and thus high diffusion rates. High temperatures also offer increased solubility of carbon in austenite-say at 1040°C, it is 1.6% C. As the steel surface become saturated with this carbon very quickly, it further increases diffusion coefficient (almost twice that at 1%C), i.e., rate of carburising increases as illustrated in Fig. 8.26.

The component to be carburised is heated to a carburising temperature of around 1040°C to 1050°C in vacuum, with a subsequent introduction of a small volume of hydrocarbon gas (1-5 torr). The component IS made cathode and is placed near the anode of a D.C. circuit. A high D C. voltage when applied ionises the gas into a glowing plasma. A thin plasma envelops the component completely reaching all its surfaces. It ionises the carburising gas and very rapidly carbon equivalent to solubility limit in austenite at that carburising temperature is adsorbed at the surface of the component.
A schematic picture of plasma furnace is illustrated in Fig. 8.27:
Plasma cycle takes only half the time of that required with vacuum carburising. Total energy consumed is 1.0:0.8 when normal gas carburising is compared to plasma carburising.
Plasma carburising has many advantages. It gives high carburising rate. Normal gas carburising profile at 900° for 240 minutes can be obtained in half the time in plasma carburising at the same temperature. Plasma carburising gives improved case uniformity even compared to vacuum carburising which is metallurgically important. It gives blind hole penetration. It is insensitive to the composition of the steel as well as to hydrocarbon gas used.
Plasma carburising provides much cleaner and safer atmosphere as there is no fire hazard or toxic gas. The operating cost is less by almost half of normal gas carburising. Individual parts in plasma carburising should not be in contact as the plasma must envelop individual part. In plasma carburising, vacuum atmosphere, makes the grain boundary oxidation resistant and decarburisation resistant. Fig. 8.27 (ii) compares the case depth of conventional gas, vacuum and plasma carburising.
7. Types of Carburising Furnaces:
Broadly the carburising furnaces can be classified into two categories:
(i) Batch type furnaces
(ii) Continuous type furnaces- These are normally preferred for carburising large tonnage of similar parts with total case depth of less than 2 mm.
Batch Type Furnaces:
These are of two main types:
(a) Pit furnaces
(b) Horizontal furnaces
Pit furnaces are put in a pit where the lid of the furnaces is located just above the floor level. These are used more commonly for large sized parts requiring deep cases (large carburising time). As after carburising, the parts are taken out of furnaces to be quenched in a tank, the hot carburised parts move in air before being quenched. The parts acquire a thin black, adherent scale on the surfaces, which can be removed later by shot- blasting, or pickling. 
The containers or fixtures containing components are loaded in the preheated furnace, which is at the operating temperature. The furnace is sealed. Also a positive furnace pressure is required so that air does not enter into the furnace which is possible in sealed-quench furnace. The flame curtain is opened to allow the additives to burn out fully. The heating is continued with the flow of carrier gas until the required temperature, normally 925°C is reached.
After attaining the carburising temperature and when the charge has been uniformly heated, calculated amount of enriching gas (10 – 15% Natural gas) is added to get around a carbon potential of 1.3%. When the required carbon content has been attained at the surface, the gas is switched off. The diffusion period begins now with the flow of carrier gas to attain a carbon of 0.8-0.9% at the surface. After the diffusion period, parts are quenched inside itself and then, these are removed.