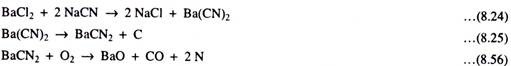In this article we will discuss about:- 1. Introduction to Carbonitriding 2. Hardenability of Case for Carbonitriding 3. Case-Depth and Concentration-Gradient 4. Retained Austenite 5. Quenching Media.
Contents:
- Introduction to Carbonitriding
- Hardenability of Case for Carbonitriding
- Case-Depth and Concentration-Gradient for Carbonitriding
- Retained Austenite for Carbonitriding
- Quenching Media for Carbonitriding
1. Introduction to Carbonitriding:
Carbonitriding (as well as cyaniding) is the case-hardening process in which both carbon and nitrogen are added to the surface layers of steel at a predetermined temperature usually in the range 800-900° for 2 to 10 hours followed by quenching. Carbonitriding is actually a modified form of carburising as the atmosphere used for it is obtained by adding 2 to 12% ammonia to the common gas-carburising atmosphere- a carrier gas (20-25% CO; 35-40% H2; 40-45% N2; 0.4-0.5% CO2; 1% H2O; 0.1-0.2% CH4) with 3.5 to 4.5% natural gas.
ADVERTISEMENTS:
Ammonia decomposes at the steel surface to give nascent-nitrogen, which is absorbed. Nitrogen diffuses into steel simultaneously with carbon.
Carbonitriding is carried out at a lower temperature (~ 100° than gas-carburising) and for a shorter time and thus, it results and is used for shallower cases-0.075 to 0.75 mm. But, the aim of carbonitriding is the same as carburising, i.e., to get a hard and wear resistant surface with tough core of the components. Normally, the steels to be carbonitrided have carbon up to 0.25%. But for some applications, like shafts and transmission gears, tough core with hard and wear resistant case (of around 0.3 mm) is required, and the steels used have carbon in the range of 0.30 to 0.50%.
Ammonia decomposes at the steel surfaces as:
NH3 Û 3/2 H2 + N(Fe) …(8.49)
ADVERTISEMENTS:
where, N(Fe) is the nitrogen dissolved at the surface of the steel.
The equilibrium constant of this reaction:
but simultaneously, ammonia at the carbonitriding temperature decomposes to:
ADVERTISEMENTS:
NH3 = 1/2 N2 + 3/2 H2 …(8.52)
Once, this molecular nitrogen is produced, it cannot be absorbed at the steel surface (nascent nitrogen, Nat is absorbed). But as this reaction (8.52) continues to occur, the partial pressure of ammonia PNH, decreases, which as per equation (8.50) leads to decrease in the potential of nitrogen in the atmosphere. Moreover, the reaction (8.52) is at different stages of completion depending on the temperature and lime. The decomposition of NH, is higher at high temperatures and low pressure of gas.
Thus, all these factors must be considered while deciding about nitrogen potential of the atmosphere. Moreover, evolution of H, and Ni by the reaction (8.52) shall affect the carbon potential of the atmosphere.
2. Hardenability of Case for Carbonitriding of Steels:
ADVERTISEMENTS:
Nitrogen is an austenite-stabiliser and also significantly increases the hardenability of case (if present in solid solution in austenite). This allows the use of those steels on which uniform case- hardness ordinarily could not be obtained, if carburising and quenching was done. Also, for many applications, the core properties are not that important but deep case (such as 2.5 mm or so) is required.
A cheap plain low carbon steel with good machinability and formability, if gas-carburised at 900- 955°C and water-quenched may not result in deep effective case-depth (martensitic transformation may not take place in the total-case due to poor hardenability of the case).
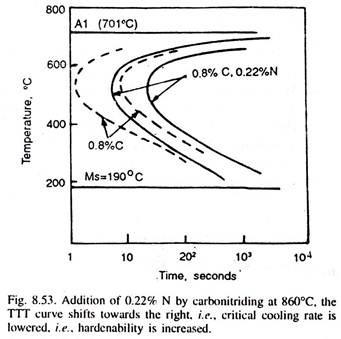
But, if carburising followed by carbonitriding at 815-900°C for 2-6 hours is done, when nitrogen (alongwith carbon) is added to the case, which improves its hardenability (Fig. 8.53), and thus, even when oil-quenched from carbonitriding temperature, it results in deeper, effective and also harder case.
Because of nitrogen addition in the case, more compressive stresses develop in the surface layers, which improve the fatigue properties. Oil-quenching and that too from a lower temperature reduces the risks of distortion of the components.
3. Case-Depth and Concentration-Gradient for Carbonitriding of Steels:
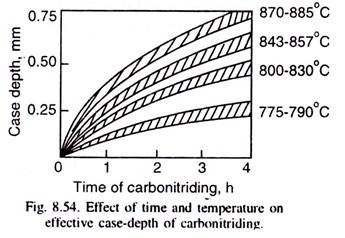
The case-depth depends on the type of steel, the temperature and time of carbonitriding as illustrated in Fig. 8.54, based on industrial data. Case-depth is lower, if the steel contains strong nitriding-forming elements like Al or Ti.
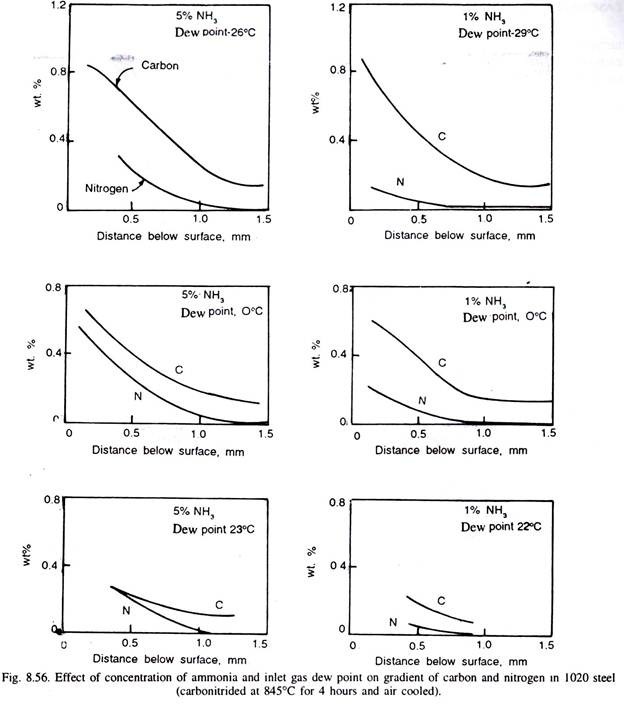
The nitrogen content of the surface depends on temperature of carbonitriding and the ammonia content of the atmosphere. Higher the temperature more is the decomposition of NH, to molecular form of nitrogen, and thus, less effective is the ammonia in the atmosphere.
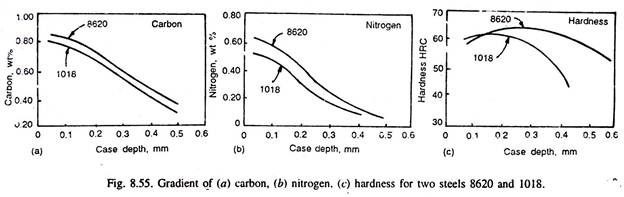
Thus, lower carbonitriding temperatures generally favour higher nitrogen concentration in the steel surface, but steeper are the nitrogen gradient, and that is why carbonitriding is done at lower temperatures than carburising temperatures. Fig. 8.55 illustrates the carbon and nitrogen content in two steels, and their gradient. Fig. 8.55 (c) illustrates variation of hardness in the carbonitrided case. Fig. 8.56 illustrates the effect of inlet gas dew point and the concentration of ammonia on carbon and nitrogen gradient in 1020 steel carbonitrided at 815°C for 4 hours.
Thin parts which require wear resistance under light loads normally require a case depth of 0.025 to 0.075 mm, but for high compressive loads, case depth up to 0.75 mm is needed.
The amount of nitrogen in the case should not be less than that required to eliminate the harmful effects of internal oxidation (i.e., at least 0.1 to 0.15% N). If the carbon content of the case is low, nodular pearlite may form on original austenite grain boundaries on quenching. Higher carbon contents in steels containing Ti, V, Cr, form carbonitride at grain boundaries as continuous or broken network. This also decreases hardenability to form nodular pearlite. Carbonitrides as well as nodular pearlite lower endurance limit, ductility and toughness of the steels.
4. Retained Austenite for Carbonitriding of Steels:
Nitrogen is an austenite stabliser and also lowers the transformation temperature of austenite. Thus, for the same carbon in case, carbonitrided case contains more retained austenite than carburised case, which is not desirable in many applications. High retained austenite content provides for good working-in properties for gears ensuring their noiseless operation in some applications. Under optimum conditions, the structure of the carbonitrided case consists of fine martensite, a small amount of fine, uniformly distributed carbide-nitride and 25-30% of retained austenite.
To reduce this retained austenite, the quenched carbonitrided parts are given sub-zero treatment by cooling further to -40°C to -100°C. It is normally more economical to choose appropriate steel (i.e., lower carbon and alloying elements), as well as control the carbonitriding process to decrease the amount of retained austenite such as by decreasing the ammonia flow (to 1 to 5%), or, if permitted to keep lower carbon potential of the atmosphere.
5. Quenching Media for Carbonitriding of Steels:
Depending on the requirement such as case depth, core hardness, and allowable distortion, the carbonitrided parts may be cooled in water/oil, or gas. Normally, carbonitriding is followed by quenching directly from the furnace, or, first cooling to 800- 825° and then quenching, or may be reheated to this temperature after slow cooling from carbonitriding temperature. Martempering is also used.
As carbonitrided parts have better hardenability, the quenching of thin parts many times may be done in cooled gas or nitrogen. Tempering is normally done at 160-180°C. Normally the hardness of case after hardening and low temperature tempering is 58 to 64 HRC (VPN 570-690).
Parts with shallow carbonitrided case, or which are primarily to resist wear may not be tempered such as dowel pins, washers, brackets, etc. Most carbonitrided gears are tempered at 190 to 205°C and still maintain case hardness of HRC 58. Tapping screws made of 1020 steel are tempered at 260° to 425°C.
Carbonitriding is preferred, over carburising, particularly for complex shapes such as toothed gears, which have a tendency to warp.
Comparing with gas carburising, carbonitriding has following advantages:
1. Lower temperatures are used (≈ 100° less)
2. Lower case depth.
3. Less distortion and warping.
4. More resistance to wear and corrosion.
5. No soot formation occurs.
Comparing it with cyaniding, the advantages are:
1. Toxic salts are not used. Hazards and pollution can be avoided.
2. Carbon and nitrogen content in the case can be controlled.
3. Medium and large size components can be processed.
4. The process is more efficient and can be mechanised.
5. This is more suited to mass and large-lot production.
Cyaniding:
Cyaniding is a case-hardening process in which both carbon and nitrogen we added to the surface layers of sic el by dipping in a liquid cyanide bath at around 800° to 900°C, specially the small parts having carbon between 0.2 to 0.4%. The main aim of cyaniding is to increase the hardness and wear resistance. The corrosion resistance as well as fatigue strength is also improved.
The typical composition of a cyaniding bath is:
NaCN = 20 – 50% (30%); NaCI = 25 – 50% (30%)
Na2 CO, = 25 – 50% (40%);
In some cases, higher cyanide content may be used.
Some of the chemical reactions are:
2 NaCN + O2 2 NaNCO …(8.16)
This cyanate then decomposes to give:
4 NaNCO→ Na2CO3 + 2 NaCN + CO + 2 N …(8.18)
2 NaNCO + O2 → Na2 CO3 + CO + 2N …(8.20)
2 CO → CO2 + C (Fe) …(8.22)
The cyanate content normally does not exceed 3%. Nascent nitrogen and carbon are absorbed by the surface of steel, which then diffuse in. Cyaniding gives higher nitrogen content (0.8 – 1.2%) and lower carbon content (0.6 to 0.7%). Sometimes, air is bubbled through the bath to accelerate the cyanate formation (reaction 8.16).
Composition of Bath for Cyaniding:
Normally the neutral salts (anhydrous Na2CO3 and NaCI) are melted first in a clean and dry pot, and the required amount of dry NaCN is then added. The neutral salts decrease the melting point and thus control the fluidity of the bath. When the bath has been melted thoroughly, its temperature is raised to the specified case-hardening temperature. A fine grade graphite powder covering is made on the bath to conserve the bath strength, decrease heat losses, prevent fumes, and also to increase the pot life.
The composition of the bath depends also on the required case- depth and the material to be cyanided. For example, for case depth up to 0.25 mm, the cyanide content in the bath is maintained at 20-28%, but for higher case depths of 0.6 mm, the bath may have cyanide content of 40-50%.
The components to be cyanided should be cleaned to be free of scale, dirt, oil, etc. and preheated to 200° to 500°C to remove moisture and conserve heat of the bath. It is then immersed in cyanide bath using suitable fixture, or basket, etc. The components are then allowed to remain in bath for 10-90 minutes, depending on the case-depth desired.
The components are then water, or oil-quenched depending on type of steel, hardened-depth, degree of distortion allowed. The cyanided mild steel is, normally water quenched. Small parts may be oil- quenched. If there is problem of distortion, then the components may be first directly quenched in oil, reheated to 780°C and then water-quenched. Selective cyaniding may be done by copper plating on area not to be cyanided, but more appropriate method is to have the machining allowance on those areas to machine off after cyaniding.
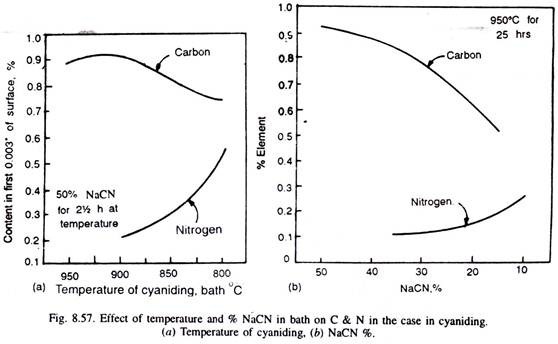
Cyaniding temperatures being low reduce distortion and warping during quenching, but the use of slightly higher temperatures enables to get greater case depths in a given time. But at higher temperatures, more carbon is enriched in the case than nitrogen (Fig. 8.57).
A temperature of around 800°C, helps to get increased nitrogen but a decreased carbon content in the case. Fig. 8.57 illustrates that with the increase of cyanide content of the bath from 10% to 50%, the carbon content of the case increases, whereas the nitrogen content decreases.
Cyanided parts after quenching are given low temperature tempering at 180 to 200°C. Normally the hardness of parts at this stage is HRc 58 to 62. Because of nitrogen content, a cyanided part has additional higher wear resistance and endurance limit than carburised part.
Cyaniding is commonly done to small parts such as, nuts, bolts, screws, small gears, gears for oil pumps & speedometer drives, rear spring pins, steering worms, small shafts, etc.
Quite often to get a deep case (0.5 to 2.00 mm), high temperature cyaniding at 900° to 960°C with working bath composition of : 8% NaCN; 82% BaCl2: 10% NaCI with graphite covering is used for 1.5 to 6 hours.
Some of the chemical reactions are:
Nascent carbons as well as nitrogen are absorbed at the steel surface which then diffuses in. Such deep cases have carbon – 0.8 – 1.2% and nitrogen = 0.2 – 0.3%. On the surface of such deep cases, a thin 0.02 to 0.03 mm layer of carbonitride e-phase forms on an almost carburised core.
The parts are normally cooled, reheated to refine the grain size and then quenched and tempered at low temperature. Deep cyaniding is replacing carburising as it requires less time for the same case depth and less warping and distortion even of intricate shapes (gears, etc.).
The components have higher wear resistance and corrosion resistance. Because of increased hardenability due to presence of nitrogen, plain carbon steels could also be used for cyaniding decreasing thereby the cost of per piece. Cyaniding is faster than carbonitriding and more flexible as simultaneously different components can be simultaneously cyanided which may require different case depths.
Cyaniding has certain disadvantages:
1. It is costly.
2. Salts used are toxic and due precautions are quite elaborate.
3. Disposal of cyanide waste is a problem,
4. There is difficulty in maintaining constant temperature due to dissociation of cyanide.