In this article we will discuss about:- 1. Introduction to Nitriding 2. Operations before Nitriding 3. Process of Nitriding 4. White Layer or Compound Layer 5. Plain Carbon Steels for Nitriding 6. Effects of Microstructure 7. Advantages and Disadvantages.
Contents:
- Introduction to Nitriding
- Operations before Nitriding
- Process for Nitriding
- White Layer or Compound Layer of Nitriding
- Plain Carbon Steels for Nitriding
- Effects of Microstructure on Nitriding
- Advantages and Disadvantages of Nitriding
1. Introduction to Nitriding:
Nitriding is a case-hardening process of enriching the solid steel surface with nitrogen at a low temperature, normally in the range of 500-575°C (i.e., below A1), when the steel is ferritic.
ADVERTISEMENTS:
There are two general types of nitriding processes:
1. For alloy steels containing strong nitride-forming elements. (Hard Nitriding)
2. For unalloyed low carbon steels. (Soft Nitriding)
Commonly, the definition of term ‘nitriding’ is synonym to gas-nitriding of nitriding (alloy) steels (also called nitralloys), i.e., it is understood as the enrichment of solid steel surface with nitrogen by heating it in an atmosphere of NH3 gas at a temperature normally in the range of 500-575°C for a prolonged period of 48 to 96 hours, depending upon the case-depth desired. Now-a-days, nitriding is also done in other mediums. The steels at the nitriding temperatures have microstructure consisting normally of ferrite and carbides.
ADVERTISEMENTS:
As NH3 dissociates to give atomic-nitrogen at the steel surface, it gets absorbed there, and then diffuses inside. There takes place interaction between N and the alloy solute atoms, principally of Al, Cr, Mo, resulting in the formation of fine, closely-spaced and uniformly dispersed coherent-alloy-nitrides precipitate-particles in ferrite. Dislocations seem to be required for the nucleation of these nitride-particles, and thus, dislocations are created providing more sites for nucleation.
The reasons of high surface hardness of around 1100 VPN in nitrided cases of these steels are:
1. Small closely—spaced nitride particles (size 5-15 nm) block the motion of dislocations.
2. As dislocations are generated, there is high density of dislocations, at least 1010 cm-2 as in heavily cold-worked metals, which increases the hardness.
ADVERTISEMENTS:
3. High concentration of N atoms forming Cottrell-type atmospheres with dislocations and also with Fe-alloy nitride interfaces.
Thus, normally the steels containing strong nitride-forming elements are case-hardened by nitriding.
As the component to be nitrided is made of expensive alloy steel, the properties of the core must be optimised before nitriding. It is invariably done by hardening and tempering. However, the tempering temperature, used to improve the properties of the core, should be at least 30°-40°C higher than the intended nitriding temperature so that no change occurs in core properties during long nitriding treatment.
As the great hardening in nitriding occurs basically by the precipitation of alloy-nitrides (not by martensitic hardening of carburised steels) and moreover nitriding is done at low temperatures when no phase-change occurs on heating, or cooling, i.e., quenching is not done to increase hardness after nitriding and thus, distortion and cracks are not encountered in nitriding.
ADVERTISEMENTS:
However, nitriding brings in some dimensional changes due to increase in volume that occurs in the case. The amount of growth can be predicted and necessary allowance should be made prior to nitriding. Due to this increased volume of the case, compressive stresses are set up in the surface layers which greatly improve the fatigue life.
Nitrided parts retain their high hardness even when used at high temperatures up to 500°C, or so, because here coagulation of fine nitride particles does not occur (which otherwise could have decreased the hardness). The hardness of carburised having the martensitic structure is retained only up to 200-225°C. That is why gears, cylinders of powerful engines are nitride. Hot-work dies after nitriding have long service life.
Main Reasons for Nitriding:
1. To obtain high surface hardness, wear resistance and antigalling properties. Hardness obtained is higher than obtained by carburising. Hardness HRC 62-67 or even 71 can be obtained.
ADVERTISEMENTS:
2. To improve fatigue properties.
3. To improve corrosion resistance in atmosphere, water, steam, etc. (except stainless steels).
4. To have good high temperature (up to nitriding temperature ≈ 550°C) properties. As the case has high resistance to tempering, it retains high hardness at high temperatures.
5. No dangers of quench cracks and distortion i.e., high dimensional stability. No other heat treatment is required after nitriding.
But, nitriding is an expensive process:
1. As low temperatures are used in nitriding, much more time is required to develop the requisite case depth. It takes 48 to 96 hours of nitriding to develop a case depth of around 1 mm.
2. Expensive gas ammonia is used in nitriding.
3. Expensive alloy steels can only be nitrided and are used.
Thus, as a case-hardening process, nitriding is more expensive as compared to carburising or carbonitriding. But now with the use of glow-discharge nitriding (ion nitriding) considerable reduction in total nitriding time (one-half or two-third) is possible.
2. Operations before Nitriding:
Normally several operations are done before nitriding:
1. Hardening and Tempering:
Nitriding steels are invariably hardened and tempered at high temperature to increase the strength and toughness of the core. The tempering temperature is at least 30° higher than the nitriding temperature, normally 600-675°C. Steel now has sorbitic structure which is also good for machining.
2. Final Machining:
All the machining operations are done which give the final size to the component, keeping a close tolerance of 0.03 mm to .05 mm on all areas as parts grow during nitriding. Sharp corners if possible should be rounded off. If heavy machining had been done, the parts may be stress-relieved by heating at 550-570°C for 2-4 hours. Final finish machining may be done then. No machining is done after nitriding.
3. Selective Nitriding:
The areas not to be nitrided are coated with a thin layer of tin (0.01-0.15 mm thick), copper or nickel or water glass. Surface tension keeps the molten tin sticking to the parts while being nitrided. Copper (electrolytically deposited) is also used quite often.
4. Then, nitriding is done as required.
5. Finish-lapping is done.
3. Process for Nitriding:
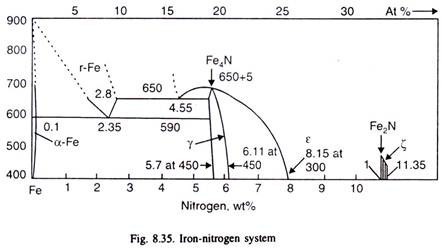
Iron-nitrogen equilibrium diagram (Fig. 8.35) can be used to study the nitriding process. At the commonly used nitriding temperature (below 590°), nitrogen dissolves in α-iron up to only 0.1% (called nitrogenous ferrite). When the nitrogen dissolved in a-iron exceeds 0.1%, next phase stable at the temperature, i.e. γ’- nitride (a solid solution) is formed. When nitrogen content exceeds about 6%, ε-nitride (a solid solution) is formed.
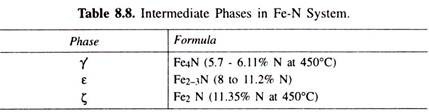
If the temperature of nitriding is below 500°C, further increase of nitrogen results in the formation of ζ-nitride which has nitrogen content of about 11%. Normally, temperatures are higher, and thus the nitrided layer has on the surface, ε-nitride, richest in nitrogen, and somewhat inside the steel, it has γ’- and then α-ferrite. Table 8.8 given these intermediate phases with chemical formula. After nitriding, as nitrided steel is cooled, surplus, γ’ is precipitated from ε and α-phases as per equilibrium diagram.
Thus, after cooling, the phases in nitrided case are in the order from surface towards the case:
ε + γ’→ γ’ → α + γ’ → α core (original structure)
Thus, plain carbon steels which don’t contain any strong nitride forming elements, when nitrided in an atmosphere of high nitrogen activity, the iron nitrides are formed. The iron nitride case thus formed is generally brittle and does not have high hardness. But steels containing strong nitride forming elements like Al, Mo, Cr, Ti, V etc. interact with diffusing-in nitrogen during nitriding and internal precipitation of their nitrides takes place resulting in high surface hardness.
Fig. 8.36 illustrates the effect of these alloying elements on hardness after nitriding a 0.35%C, 0.30% Si, 0.70% Mn steel. Table 8.9 illustrates the nitrides of these alloying elements.
Thus, while deciding about the composition of the nitriding steel, the choice falls on elements which form nitrides such as Al, Cr, Mo. In Ni steel, nitriding results in same hardness as in plain carbon steels (≈ 400 VPN). Steels containing several alloying elements result in much higher hardness values than by a single element.
Elements like Al and Ti have strong tendency particularly Ti to reduce case depth because these bind the nitrogen as nitride. The optimum content is 1% Al, which is the amount normally present in nitriding steels. Carbon too has a strong inhibiting effect on the diffusion of nitrogen. Table 8.10 given composition of nitriding steels.
Of the three factors responsible for increased surface hardness after nitriding, the factor precipitation of fine nitride particles depends on the size, distribution and volume fraction of the nitride particles. The formation of alloy nitride precipitates during nitriding involves the diffusion of nitrogen from the surface and the diffusion of alloying elements in the ferrite matrix.
At the nitriding temperatures, the diffusivity of nitrogen, which is an interstitial solid solution forming element, is orders of magnitude faster than that of the alloying elements, which are substitutional solid solution forming elements. Thus, the extent of alloy nitride precipitation is controlled by the diffusion of nitrogen the precipitate size is controlled essentially by the diffusion of the alloying elements.
The volume fraction of the nitride precipitates is proportional to the amount of the nitride forming alloying elements present in the nitriding steels. The diffusion of substitutional solid solution forming elements in steels at around 500-575°C is very slow and thus, the precipitate size is sufficiently (5-15 nm) fine to result in high precipitation hardening effects.
Thus, to obtain high hardness in nitrided case, the volume fraction of the precipitates is increased by increasing concentration of the alloying element, preferably a number of alloying elements. The size of the precipitate is kept low by using lower nitriding temperatures. Case depth increases with the increase of nitriding temperature but maximum hardness decreases as illustrated in Fig. 8.37.
4. White Layer or Compound Layer of Nitriding:
Fig. 8.39 (a) illustrates cross section of nitrided part, polished and nital etched, whereas Fig. 8.39 (b) illustrates this schematically. Nitrided case invariably contains a compound zone on the surface of the part apart from the diffusion zone. The outer compound zone is called white layer due to its colour under microscope after polishing and nital- etching. This layer consists normally of γ’ (Fe4N) and ε (Fe2-3N) intermetallics (apart from nitrides of alloying elements).
See Fig. 8.35. When the nitrogen potential, as happens in most industrially nitriding, of the nitriding atmosphere is higher than the solubility limit of nitrogen in iron, γ’ (Fe4N) forms, and at still higher potential of nitrogen, ε (Fe2-3N) also forms.
The incubation period for the formation of γ’-Fe4N is large and does not form easily however, ε (Fe2-3N) forms easily at the surface and grows with time. γ(Fe4N) may then nucleate below ε and grow. In an atmosphere of high nitrogen activity, the nitriding rate is relatively faster till Fe4N is formed. Once Fe4N has formed, the nitrogen potential at this surface falls.
The white layer during gas nitriding consists invariably of a mixture of Y and e compounds; the mixture is due to variability of dissociation of ammonia, and therefore of nitrogen potential as the compound layer is formed. Normally both phases exist throughout the white layer -also called dual-phase layer. The white layer may form to depths upto 0.05 mm on the outer surface of the nitrided parts.
This white layer is generally brittle and tends to chip and may spall under service conditions leading to seizure and/or cracking. The presence of cracks greatly reduces the fatigue life of the components. This white layer is not harmful if its thickness is less than 0.01 mm, but if it is more, it is undesirable. It must be either completely removed, or greatly reduced before the nitrided part is put to use in service.
The white layer may be removed by grinding, lapping or by chemical dissolution. Grinding or lapping can be done to only relatively smooth surfaces. The chemical dissolution is more widely used, which consists of soaking the nitrided parts in hot alkaline solution (100 gram of NaCN in 1 liter of water heated to 75-90°C) for short periods, which makes the white layer porous and friable. This layer can be polished off by Al2O3 of 220 mesh grit at 550 kN/nr pressure.
The total soaking time may be up to 8 hours. This method is very cheap to free the surfaces of white layer completely. This method simultaneously strips copper which might have been applied for selective nitriding. Other methods like vapour blasting, or blast cleaning may be used. The two-step (Floe process) gas nitriding reduces this white layer. Ion nitriding also reduces this layer. The use of mixture of NH3 and H2 with an activity of nitrogen less than that required to form Fe4N at the temperature also prevents white layer formation.
The inside-diffusion-zone is basically the original core microstructure with formation of some solid solution and precipitation of coherent nitrides (of iron as well as of alloying elements). The precipitates are inside the grain as well as at grain boundaries. In most ferrous alloys, the diffusion zone cannot be seen in a metallograph, because coherent precipitates are too small to be resolved.
ε layer is best for wear and fatigue resistance. γ’ layers is a bit softer, less wear resistant but is tougher and good in severe loading conditions. White layer provides increased lubricity. As white layer is inert, it provides increased corrosion resistance in many atmospheres.
5. Plain Carbon
Steels for Nitriding:
Plain carbon steels, on nitriding develop iron nitride layer which is brittle, and thus are not suitable for case-hardening by nitriding. However, plain carbon steels are nitrided for improving scuffing, wear and corrosion resistance.
Steels to be nitrided for case-hardening should contain strong nitride forming elements to develop hard and wear resistant nitrided cases. Fig. 8.36 illustrates the effect of some of the strong nitride forming alloying elements. Thus, nitriding steels must contain some of the elements such as Al, Cr, Mo, V, Ti, etc.
The surface hardness developed by nitriding depends on the nature, and amount of nitride forming elements-preferably a number of the elements present simultaneously than a single element in large amount Low-alloy steels having Cr-Mo such as 41 XX, 86 XX, etc. having 1-2% Cr and 0.5% Mo yield a hardness value of 40-50 HRc. Mo also reduces temperbrittleness.
Steels containing Al, Cr, Mo attain high hardness of 70-71 HRc. Some tool steels, stainless steels, and even cast irons having nitride forming elements can be nitrided as well.
Soft Nitriding-Nitriding of Plain Carbon Steels:
Surface properties of plain carbon steels can be improved by nitriding of plain carbon steels. Improvement in properties like wear resistance under small loads, the maximum resistance to scuffing and galling at lowest cost and even corrosion resistance in moist atmospheres, or in NaCl solutions, when the solutions are not acidic, takes place. The nitriding may be done by liquid baths or gas nitriding for 2 to 4 hours at 500- 570°C. Fatigue properties are also improved.
When plain carbon steels are nitrided for 2 to 4 hours, surface gets an iron nitride compound layer and a nitrogen diffusion zone below it. The compound layer is generally less than 20 µm thick and consists mainly of ε nitride. The thickness of diffusion zone could be 1 mm. After nitriding, the parts are invariably oil, or water quenched to retain nitrogen in solid solution, which maintains compressive stresses in surface layers to increase the fatigue life. Fig. 8.52 illustrates the hardness distribution in such nitrided case.
Fig.8.52. Hardness distribution in nitrided case of plain carbon steel. Low hardness at the surface is due to porous nature of layer.
Gas nitriding producing ε nitride or carbonitride compound layer can be done in:
(i) NH3,
(ii) NH3-N2,
(iii) NH3- endothermic gas (40% N2, 20% CO, 40% H2 traces of CO2),
(iv) NH3-N2– CO2 mixtures.
The presence of CO or CO2 in nitriding atmosphere accelerates the nitriding reaction. CO2, which is either present or produced by reaction- 2 CO à C + CO2, reacts with H2 obtained by dissociation of NH3, increases this rate of dissociation of NH3. A thickness of compound layer of about 0.018 mm is obtained by nitriding for 2 hours at 570°C, and which is cheapest way to have maximum resistance to scuffing. ε-nitride has a hardness of 1100 on knoop scale.
6. Effects of Microstructure on Nitriding:
If the microstructure contains large amount of free ferrite, the diffusion of nitrogen is faster. Looking at it the other-way, when the amount of carbide is low diffusion of nitrogen is faster and high hardness may result.
All the nitride forming elements except Al also form alloy carbides. These either segregate preferentially in cementite or form their carbides. Steels to be nitrided are invariably quenched and tempered to improve properties of the core. The tempering temperature is always 30°C higher than the nitriding temperature.
Thus, depending on the time and temperature of this tempering, the alloy nitriding steels could have in microstructure:
(i) Ferrite and alloyed-cementite, or
(ii) Ferrite and alloy carbides
The nitride forming alloying elements are preferentially segregated, in cementite in the first case. Alloy nitride is more stable than cementite. Thus, during nitriding as nitrogen diffuse inside, the cementite decomposes the alloying element now dissolves easily in the adjacent ferrite. The alloying element interacts with nitrogen to precipitate alloy nitride particles.
Carbon too dissolves and diffuses away from the nitriding interface but precipitates either as cementite or as alloy carbide ahead of the interface. Thus, the kinetics of nitriding is then dependent on the decomposition kinetics of cementite. If the cementite had grown in size by coagulation during tempering, the kinetics of nitriding becomes slow because the alloying element must diffuse over longer distances.
The second case is more common, i.e. prior tempering has resulted in alloying elements present as alloy carbides, which also might have coagulated. Alloy carbides are definitely less stable than alloy nitrides but more stable than cementite and, also contains more amount of the alloying elements.
Thus, all the alloy carbides must dissolve completely to free the alloying elements to form nitrides. The rate of dissolution of alloy carbides is much slower than that of cementite, and thus, the kinetics of nitriding becomes further very slow.
If the prior tempering is done at very high temperature, alloy carbides precipitate and coagulate to become large sized and far-spaced. Diffusion of alloying elements over larger distances in required to form alloy nitrides which makes the process further slower.
It is possible that alloy carbides may not dissolve completely (because of their being large sized) which results in lesser hardness as is illustrated in Fig. 8.38 as alloying elements trapped as undissolved carbides are not able to form their hard nitride particles.

There is another reason of slowing down of nitriding process. Alloy carbides (during tempering) precipitate preferentially at the grain boundaries. Diffusion of alloying elements takes place more rapidly along grain boundaries than through the grains.
These carbide precipitates act as barriers to the diffusion, and when nitrides or carbonitrides form, these too are present at the grain boundaries to slow down the process of diffusion, i.e., of nitriding ultimately. If the prior tempering temperature had been close to nitriding temperature, then the tempering process can also continue taking place even during nitriding, which complicates the process of nitriding.
7. Advantages and Disadvantages of Nitriding:
1. Case-Depth:
Salt-bath nitriding is commonly restricted to 4 hours, because the density of pores increases with time. Gas nitriding is not restricted but normally a practically reasonable time of 90- 98 hours is not exceeded. Thus, when greater-depth than that can be obtainable with salt-bath nitriding is required, gas-nitriding is done.
2. Time:
Comparing for a case-depth of around 0.1 mm (at hardness of 400 VPN for a steel), salt bath takes much less time of around 2 hours than gas-nitriding.
3. Dimensional distortion is the same (for the same case depth) in both cases, but because salt-bath- nitrided components are water or oil-quenched, additional stresses result in changes in shape, which is extra than obtained in gas-nitriding.
4. Wear Resistance:
Salt-bath nitriding results in higher content of e-carbonitride than in gas-nitriding, unless hydrocarbon is added in nitriding atmosphere to result in same amount of ε -carbonitride. Higher content of ε -carbonitride provides more wear resistance and reduced risk of scuffing.
5. Toughness:
The toughness of the nitrided case by salt-bath nitriding is inferior to that obtained by gas-nitriding due to rapid quenching done after salt-bath nitriding.
6. Cleanliness:
Gas-nitriding is more clean job than salt bath nitriding particularly the related problems of using cyanide salts. There is always a danger even if all the precautions are observed with salt baths.
7. For smaller case depths, salt bath nitriding is cheaper as it takes lesser time.