In this article we will discuss about the effect of alloying elements on the properties of steel.
General Effect of Alloying Elements:
It has already been seen that both ferrite and cementite will be present in the carbon steel in equilibrium at room temperature; the ferrite being a soft constituent and cementite a hard one. Further it is the shape and distribution of the carbides in the iron (and not the mere presence of cementite) that causes steel to be hard.
It has also been seen that it is possible to dissolve all the carbide in the austenite by heating steel in the region GSEN and then obtain the desired size and distribution of carbides in the ferrite by suitable cooling through the cooling range (i.e. proper heat-treatment).
If carbon content in steel is equal to 0.8% (eutectoid composition), its slow cooling through the critical temperature produces a structure known as pearlite having alternate plates of ferrite and cementite (because both of these constituents are rejected simultaneously in this case).
ADVERTISEMENTS:
If carbon is less than 0.8% (hypo-eutectoid composition) then first free ferrite is rejected till the composition of the remaining austenite reaches 0.80% carbon when the simultaneous rejection of both ferrite and carbide will again occur, thus resulting in a structure consisting of areas of free ferrite and areas of pearlite.
When carbon content is more than 0.8% (hypereutectoid composition) and such a steel is cooled slowly through the critical range than cementite is thrown out at the austenite grain boundaries, forming a cementite network until the austenite containing 0.8% carbon at which time pearlite is again formed; the resulting structure thus being composed of areas of pearlite surrounded by a thin carbide network.
If the cooling rate is high then the spacing between the pearlite lamellae becomes smaller and there is greater dispersion of carbide which prevents slip in the iron crystals and makes the steel hard. When the cooling rate is very high (quenching), the carbon does not have sufficient time to separate out in the form of carbide, and the austenite transforms to a highly stressed structure supersaturated with carbon called martensite which is very hard and brittle.
When this martensite is heated to some temperature below the critical, the carbide precipitates out in the form of small spheroids (the size of which depends directly on the temperature) and the resulting structure becomes ductile and hardness is lowered.
ADVERTISEMENTS:
Although the characteristic behaviour of carbon steel is obliterated by addition of alloying elements (which are added to modify the properties of carbon steel to an appreciable extent) but they still owe their distinctive characteristics to the carbon contained.
Any steel always contains only two constituents, i.e. ferrite and carbide. The only way the alloying elements can affect the properties of steel is to change the dispersion of carbide in the ferrite, change the properties of the ferrite, or change the properties of the carbide.
With plain carbon steels it is not possible to attain uniform hardness throughout the cross-section of large specimen even with critical quenching mediums. The hardenability of plain carbon steels can be increased by addition of alloying elements without resorting to drastic quenching operations which may otherwise lead to cracking and warping.
Elements such as molybdenum, tungsten, and vanadium are present as carbides in the austenite and prevent the agglomeration of carbides in tempered martensite. The presence of these stable carbide-forming elements enables higher tempering temperatures to be used without sacrificing strength. This permits these alloys to have a greater ductility for a given strength than plain carbon steels.
ADVERTISEMENTS:
The presence of alloying elements like phosphorus, silicon, manganese, nickel, molybdenum, tungsten, and chromium in the solid solution of ferrite increases the strength of metal. Aluminium influences the austenite grain size. Martensite formed from a fine grained austenite has considerably greater resistance to shock than when formed from a coarse-grained austenite.
The oxides formed by deoxidation of the steel by different elements apparently prevent grain growth about the critical temperature over a considerable temperature range. The presence of finely scattered carbides in the austenite appears to have a similar effect on the austenite grain size, so the elements forming such stable carbides will also contribute to the formation of a fine grained austenite.
The reasons for alloying various elements in the steel are given below:
(i) To delay the rate at which austenite is transformed to pearlite upon quenching to allow sufficient time for thick sections to be hardened throughout (without the use of a quench that is so drastic as to induce damaging cracks in the steel).
ADVERTISEMENTS:
The metals contributing this effect are Mn, Cr, Mo, W, Ni, Si.
(ii) To provide higher hardness at high temperature (Mo, Cr, V, W).
(iii) To provide higher strength at elevated temperatures (Mn, Ni, Si, C, Cr, Mo).
(iv) To inhibit grain growth in austenite during heat treatment (V, Al).
ADVERTISEMENTS:
(v) To provide corrosion resistance, (Cr, Ni).
(vi) To provide additional hard abrasive particles to improve wear resistance (V, Mo, Cr, W).
(vii) To combine with oxygen to prevent blowholes (Si, Al, Ti).
(viii) To combine with sulphur which otherwise causes brittleness (Mn).
(ix) To improve machining properties (S, P, Pb).
In general W, Mo, V and Cr achieve above properties by forming carbides that are insoluble in ferrite; Si, Mn, Ni, Co and Cr by going into solution in the ferrite; Si, Al, Ti, Mn, S, P, Pb help in achieving these properties by forming inclusions which are insoluble in ferrite.
Effect of Individual Elements:
(i) Nickel:
It is soluble in all proportions in both alpha and gamma forms of iron. It strengthens and toughens the ferrite phase. It imparts elasticity, hardness, and fatigue resistance to steel. It lowers the transformation points and eutectoid composition. It lessens distortion in quenching and improves corrosion resistance.
If carbon content is low, then about 3% nickel is sufficient to make steel tough. If steel is to be tough and respond to oil-quenching, then upto 5% nickel is used. If nickel quantity is too much then martensite will form too readily during quenching, and the steel will be too brittle and hard. Case-hardening steels containing nickel do not suffer grain growth during carburising and do not always require refining treatment.
Certain stainless and heat-resisting steels are produced by adding about 8% nickel with chromium, because these two elements prevent the breakdown of austenite during cooling to room temperature. Nickel is often included as alloying element in heavy forgings and high-strength structural steels that harden by air cooling rather than by quenching in oil.
(ii) Chromium:
It forms a complex series of chromium carbides in steel thus improving the depth to which a metal may be hardened and increasing its resistance to abrasion and wear. These carbides are very hard. Chromium improves wear and cutting ability. It raises A3 transformation and lowers the carbon content of eutectoid composition.
Combination of nickel and chromium is used to improve mechanical properties of steel.
Chromium counteracts the tendency of nickel to graphitise steel by stabilising the iron carbide and as such both Cr and Ni are found together in steels. Chromium tends to promote coarse-gain structure and increases the difficulty of heat treatment, and this is counteracted by nickel which refines the grain size.
Corrosion-resisting and heat resisting steels contain very high percentage of chromium.
(iii) Molybdenum:
It can form solid solution in the ferrite phase of steels. It can also form complex carbides with certain ratios of carbon to molybdenum. It raises the A3 transformation point. Molybdenum also joins with carbon and promotes hardenability.
Steels containing chromium and nickel suffer from temper brittleness i.e. they become brittle if held at a temperature between 250—500°C and as such their shock resistance becomes poor at these temperatures. This trouble can be overcome by adding about 0.25% molybdenum. Addition of molybdenum also hampers grain growth in steel at high temperature, making the steel finer grained and tougher.
(iv) Manganese:
It is an alloying element when its quantity present is more than the required quantity for de-oxidation purposes. It forms manganese carbide. It is soluble in alpha and gamma iron. It increases the depth of hardness. Steels containing 1 to 1.5% carbon and 11 to 14% manganese are resistant to wear, work harden easily, and are resistant to abrasion under shock. It counteracts the effects of sulphur.
(v) Silicon:
It is considered an alloying element when its quantity exceeds the quantity required for de-oxidation. Silicon is soluble in ferrite component. When it is used up to 2.5%, it increases the strength without decreasing ductility.
(vi) Vanadium:
It forms carbides with steels. It gives strength and toughness to steel. It improves the hardening quality of steel. It raises the transformation points and lowers the carbon content of eutectoids. It is used for providing a fine-grained structure over a broad range of temperature.
(vii) Boron:
Boron is added in quantities varying from 0.0005 to 0.001%. It improves hardness and mechanical properties of steel. It also improves rolling qualities of steel.
(viii) Aluminium:
It is used as effective de-oxidiser. Its addition controls grain growth. In nitriding steels it is used from 0.9 to 1.5% for surface hardening due to the formation of stable aluminium nitride.
(ix) Tungsten:
It increases cutting hardness and magnetic retentivity. It also causes the refinement of grains. It raises the softening temperature to about 600°C. 18% tungsten is used in high speed steel, a cutting tool material.
(x) Copper:
It is added (0.2 to 0.5%) to increase atmospheric corrosion resistance and also as strengthening agent.
(xi) Phosphorus:
In low carbon steels, it improves machinability.
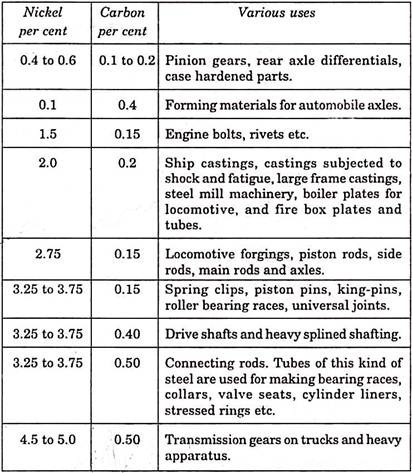
Table below shows uses of various nickel chromium low alloy-steels:
According to BIS, five types of commonly used steel castings are designated as under:
(a) Unalloyed steel castings—CS followed by minimum tensile strength in kg/mm2.
(b) Unalloyed special and steel castings like high magnetic permeability—CSM followed by minimum tensile strength in kg/mm2.
(c) Alloy steel castings—CS followed by important alloying contents and their amount indicated in percentages after the minimum tensile strength value.
(d) Heat resistant steel castings—CSH.
(e) Corrosion resistance steel castings—CSC.
Both (d) and (e) are followed by minimum tensile strength and alloying elements contents.